|
Nuclear Forensics
Nuclear forensics is the investigation of nuclear materials to find evidence for the source, the trafficking, and the enrichment of the material. The material can be recovered from various sources including dust from the vicinity of a nuclear facility, or from the radioactive debris following a nuclear explosion. Results of nuclear forensic testing are used by different organisations to make decisions. The information is typically combined with other sources of information such as law enforcement and intelligence information. History The first seizures of nuclear or otherwise radioactive material were reported in Switzerland and Italy in 1991. Later, reports of incidents of nuclear material occurred in Germany, the Czech Republic, Hungary and other central European countries. Nuclear Forensics became a new branch of scientific research with the intent of not only determining the nature of the material, but also the intended use of the seized material as well as its origin and about ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Materials
Nuclear material refers to the metals uranium, plutonium, and thorium, in any form, according to the IAEA. This is differentiated further into "source material", consisting of natural and depleted uranium, and "special fissionable material", consisting of enriched uranium ( U-235), uranium-233, and plutonium-239. Uranium ore concentrates are considered to be a "source material", although these are not subject to safeguards under the Nuclear Non-Proliferation Treaty. According to the Nuclear Regulatory Commission(NRC), there are four different types of regulated nuclear materials: special nuclear material, source material, byproduct material and radium. Special nuclear materials have plutonium, uranium-233 or uranium with U233 or U235 that has a content found more than in nature. Source material is thorium or uranium that has a U235 content equal to or less than what is in nature. Byproduct material is radioactive material that is not source or special nuclear material. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide Fluoride
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare-earth elements or rare-earth metals. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. There is some dispute on whether lanthanum or lutetium is a d-block element, but lutetium is usually considered so by those who study the matter; it is included due to its chemical similarities with the other 14. All lanthanide elements form trivalent cations, Ln3+, whose chemistry is largely determined by the ionic radius, which decreases steadily from lanthanum to lutetium. These elements are called lanthanides because the elements in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parts Per Billion
In science and engineering, the parts-per notation is a set of pseudo-units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, they are pure numbers with no associated units of measurement. Commonly used are parts-per-million (ppm, ), parts-per-billion (ppb, ), parts-per-trillion (ppt, ) and parts-per-quadrillion (ppq, ). This notation is not part of the International System of Units (SI) system and its meaning is ambiguous. Overview Parts-per notation is often used describing dilute solutions in chemistry, for instance, the relative abundance of dissolved minerals or pollutants in water. The quantity "1 ppm" can be used for a mass fraction if a water-borne pollutant is present at one-millionth of a gram per gram of sample solution. When working with aqueous solutions, it is common to assume that the density of water is 1.00 g/mL. Therefore, it is common to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Detection Limit
The limit of detection (LOD or LoD) is the lowest signal, or the lowest corresponding quantity to be determined (or extracted) from the signal, that can be observed with a sufficient degree of confidence or statistical significance. However, the exact threshold (level of decision) used to decide when a signal significantly emerges above the continuously fluctuating background noise remains arbitrary and is a matter of policy and often of debate among scientists, statisticians and regulators depending on the stakes in different fields. Significance in analytical chemistry In analytical chemistry, the detection limit, lower limit of detection, or LOD (limit of detection), often mistakenly confused with the analytical sensitivity, is the lowest quantity of a substance that can be distinguished from the absence of that substance (a '' blank value'') with a stated confidence level (generally 99%). The detection limit is estimated from the mean of the blank, the standard deviation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Ion Mass Spectrometry
Secondary-ion mass spectrometry (SIMS) is a technique used to analyze the composition of solid surfaces and thin films by sputtering the surface of the specimen with a focused primary ion beam and collecting and analyzing ejected secondary ions. The mass/charge ratios of these secondary ions are measured with a mass spectrometer to determine the elemental, isotopic, or molecular composition of the surface to a depth of 1 to 2 nm. Due to the large variation in ionization probabilities among elements sputtered from different materials, comparison against well-calibrated standards is necessary to achieve accurate quantitative results. SIMS is the most sensitive surface analysis technique, with elemental detection limits ranging from parts per million to parts per billion. History In 1910 British physicist J. J. Thomson observed a release of positive ions and neutral atoms from a solid surface induced by ion bombardment. Improved vacuum pump technology in the 1940s enabled the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Ionization Mass Spectrometry
Thermal ionization mass spectrometry (TIMS) is also known as surface ionization and is a highly sensitive isotope mass spectrometry characterization technique. The isotopic ratios of radionuclides are used to get an accurate measurement for the elemental analysis of a sample. Singly charged ions of the sample are formed by the thermal ionization effect. A chemically purified liquid sample is placed on a metal filament which is then heated to evaporate the solvent. The removal of an electron from the purified sample is consequently achieved by heating the filament enough to release an electron, which then ionizes the atoms of the sample. TIMS utilizes a magnetic sector mass analyzer to separate the ions based on their mass to charge ratio. The ions gain velocity by an electrical potential gradient and are focused into a beam by electrostatic lenses. The ion beam then passes through the magnetic field of the electromagnet where it is partitioned into separate ion beams based on the io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma-ray Spectrometer
A gamma-ray spectrometer (GRS) is an instrument for measuring the distribution (or spectrum—see figure) of the intensity of gamma radiation versus the energy of each photon. The study and analysis of gamma-ray spectra for scientific and technical use is called gamma spectroscopy, and gamma-ray spectrometers are the instruments which observe and collect such data. Because the energy of each photon of EM radiation is proportional to its frequency, gamma rays have sufficient energy that they are typically observed by counting individual photons. Gamma-ray spectroscopy Atomic nuclei have an energy-level structure somewhat analogous to the energy levels of atoms, so that they may emit (or absorb) photons of particular energies, much as atoms do, but at energies that are thousands to millions of times higher than those typically studied in optical spectroscopy. (Note that the short-wavelength high-energy end, of the atomic spectroscopy energy range (few eV to few hundred keV), ge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma Rays
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (), it imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation ''gamma rays'' based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power. Gamma rays from radioactive decay are in the energy range from a few kiloelectronvolts ( keV) to approximately 8 megaelectronvolts ( MeV), corresponding to the typical energy levels in nuclei with reasonably long ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-particle Spectroscopy
Alpha spectrometry (also known as alpha(-particle) spectroscopy) is the quantitative study of the energy of alpha particles emitted by a radioactive nuclide that is an alpha emitter. As emitted alpha particles are mono-energetic (i.e. not emitted with a spectrum of energies, such as beta decay) with energies often distinct to the decay they can be used to identify which radionuclide they originated from. Experimental methods Counting with a source deposited onto a metal disk It is common to place a drop of the test solution on a metal disk which is then dried out to give a uniform coating on the disk. This is then used as the test sample. If the thickness of the layer formed on the disk is too thick then the lines of the spectrum are broadened to lower energies. This is because some of the energy of the alpha particles is lost during their movement through the layer of active material. Liquid scintillation An alternative method is to use liquid scintillation counting (LSC), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
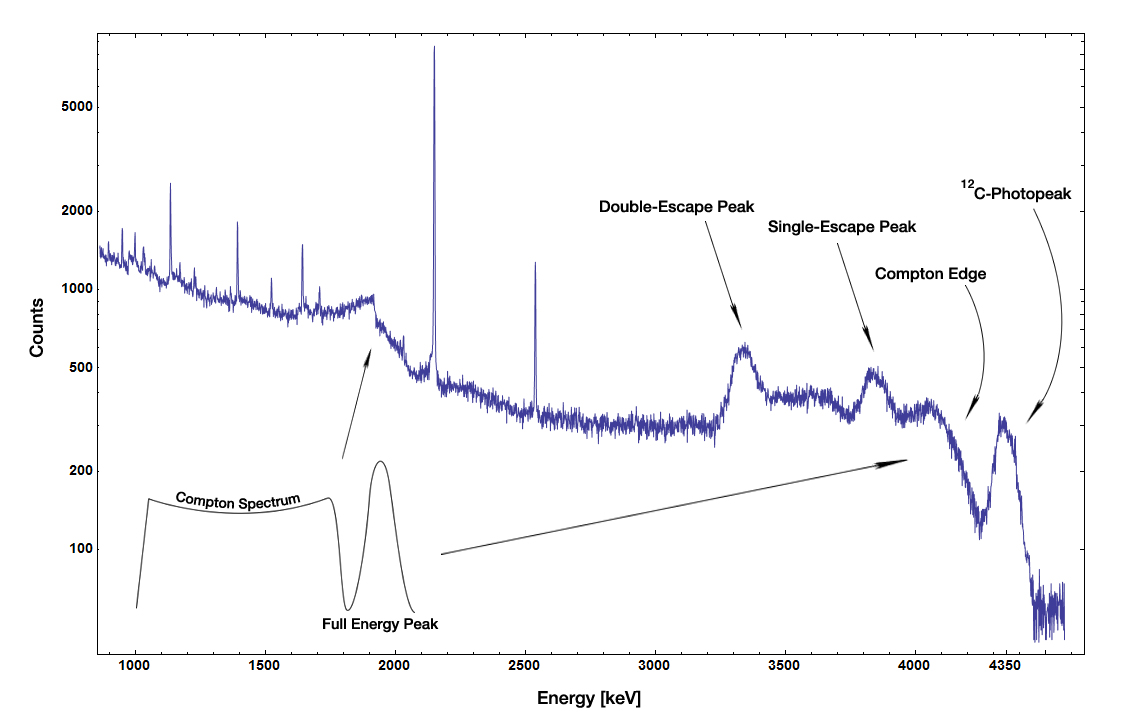
Gamma Spectroscopy
Gamma-ray spectroscopy is the quantitative study of the energy spectra of gamma-ray sources, such as in the nuclear industry, geochemical investigation, and astrophysics. Most radioactive sources produce gamma rays, which are of various energies and intensities. When these emissions are detected and analyzed with a spectroscopy system, a gamma-ray energy spectrum can be produced. A detailed analysis of this spectrum is typically used to determine the identity and quantity of gamma emitters present in a gamma source, and is a vital tool in radiometric assay. The gamma spectrum is characteristic of the gamma-emitting nuclides contained in the source, just like in an optical spectrometer, the optical spectrum is characteristic of the material contained in a sample. Gamma ray characteristics Gamma rays are the highest-energy form of electromagnetic radiation, being physically the same as all other forms (e.g., X-rays, visible light, infrared, radio) but having (in general) high ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an atomic number that is reduced by two. An alpha particle is identical to the nucleus of a helium-4 atom, which consists of two protons and two neutrons. It has a charge of and a mass of . For example, uranium-238 decays to form thorium-234. While alpha particles have a charge , this is not usually shown because a nuclear equation describes a nuclear reaction without considering the electrons – a convention that does not imply that the nuclei necessarily occur in neutral atoms. Alpha decay typically occurs in the heaviest nuclides. Theoretically, it can occur only in nuclei somewhat heavier than nickel (element 28), where the overall binding energy per nucleon is no longer a maximum and the nuclides are therefore unstable toward spont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)