|
Norpsilocin
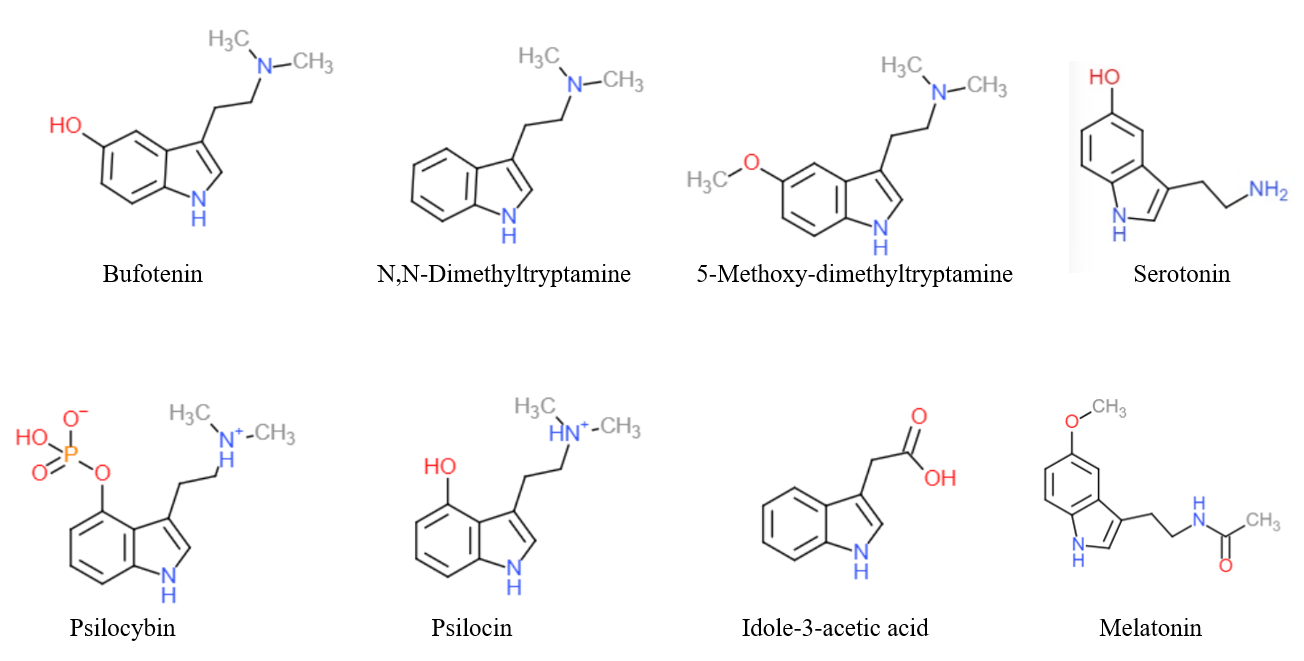
Norpsilocin (4-HO-NMT) is tryptamine alkaloid recently discovered in 2017 in the psychedelic mushroom ''Psilocybe cubensis''. It is hypothesized to be a dephosphorylated metabolite of baeocystin. Norpsilocin was found to be a full agonist of 5-HT2A receptor. It is also more potent than psilocin. See also * Norbaeocystin Norbaeocystin is a psilocybin mushroom alkaloid and analog of psilocybin. It is found as a minor compound in most psilocybin mushrooms together with psilocin, psilocybin, aeruginascin, and baeocystin, from which it is a derivative. Norbaeocystin ... References Tryptamine alkaloids Secondary amines {{Alkaloid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norbaeocystin
Norbaeocystin is a psilocybin mushroom alkaloid and analog of psilocybin. It is found as a minor compound in most psilocybin mushrooms together with psilocin, psilocybin, aeruginascin, and baeocystin, from which it is a derivative. Norbaeocystin is a N-demethylated derivative of baeocystin (itself a N-demethylated derivative of psilocybin), and a phosphorylated derivative of 4-hydroxytryptamine. The latter is notable as a positional isomer of serotonin, which is 5-hydroxytryptamine. See also * Aeruginascin * Baeocystin * Norpsilocin * Psilocybin Psilocybin ( , ) is a naturally occurring psychedelic prodrug compound produced by more than 200 species of fungi. The most potent are members of the genus ''Psilocybe'', such as '' P. azurescens'', '' P. semilanceata'', and '' P.&nbs ... References Tryptamine alkaloids Organophosphates Psychedelic tryptamines {{hallucinogen-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baeocystin
Baeocystin is a zwitterionic alkaloid and analog of psilocybin. It is found as a minor compound in most psilocybin mushrooms together with psilocybin, norbaeocystin, aeruginascin, and psilocin. Baeocystin is an ''N''-demethylated derivative of psilocybin, and a phosphorylated derivative of 4-HO-NMT (4-hydroxy- ''N''-methyltryptamine). The structures at right illustrate baeocystin in its zwitterionic form. Baeocystin was first isolated from the mushroom ''Psilocybe baeocystis'', and later from '' P. semilanceata'', '' Panaeolus renenosus'', ''Panaeolus subbalteatus'', and '' Copelandia chlorocystis''. It was first synthesized by Troxler ''et al''. in 1959. Little information exists with regard to human pharmacology, but in the book ''Magic Mushrooms Around the World'', author Jochen Gartz reports being aware of a study in which "10 mg of baeocystin were found to be about as psychoactive as a similar amount of psilocybin." Gartz also reported in a research paper that a self ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.Chemical Encyclopedia: alkaloids xumuk.ru Alkaloids are produced by a large variety of organisms including , , Medicinal plant, plants, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psychedelic Mushroom
Psilocybin mushrooms, commonly known as magic mushrooms, are a polyphyletic informal group of mushroom, fungi that contain psilocybin which turns into psilocin upon ingestion. Biological genera containing psilocybin mushrooms include ''Psilocybe'', ''Panaeolus'' (including ''Copelandia''), ''Inocybe'', ''Pluteus'', ''Gymnopilus'', and ''Pholiotina''. Psilocybin mushrooms have been and continue to be used in indigenous New World cultures in religious, Divination, divinatory, or Spirituality, spiritual contexts. Psilocybin mushrooms are also used as recreational drugs. They may be depicted in Stone Age rock art in Africa and Europe, but are most famously represented in the Pre-Columbian sculptures and glyphs seen throughout North, Central and South America. History Early Prehistoric rock arts near Villar del Humo in Spain, suggests that ''Psilocybe hispanica'' was used in religious rituals 6,000 years ago. The hallucinogenic species of the Psilocybe genus have a history of us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psilocybe Cubensis
''Psilocybe cubensis ''is a species of psychedelic mushroom whose principal active compounds are psilocybin and psilocin. Commonly called shrooms, magic mushrooms, golden halos, cubes, or gold caps, it belongs to the fungus family Hymenogastraceae and was previously known as ''Stropharia cubensis''. It is the best-known psilocybin mushroom due to its wide distribution and ease of cultivation. Taxonomy and naming The species was first described in 1906 as ''Stropharia cubensis'' by American mycologist Franklin Sumner Earle in Cuba. In 1907, it was identified as ''Naematoloma caerulescens'' in Tonkin (now northern Vietnam) by French pharmacist and mycologist Narcisse Théophile Patouillard, while in 1941, it was called ''Stropharia cyanescens'' by William Alphonso Murrill near Gainesville in Florida. German-born mycologist Rolf Singer moved the species into the genus ''Psilocybe'' in 1949, giving it the binomial name ''Psilocybe cubensis''. The synonyms were later also as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Natural Products
The ''Journal of Natural Products'' is a monthly peer-reviewed scientific journal covering all aspects of research on the chemistry and/or biochemistry of naturally occurring compounds. It is co-published by the American Society of Pharmacognosy and the American Chemical Society. The editor-in-chief is Philip J. Proteau (Oregon State University). History The journal was established in 1938 as ''Lloydia'', published by the Lloyd Library and Museum, and obtained its present title in 1979. It has been the official journal of the American Society of Pharmacognosy since 1961. Originally a quarterly publication, it became a bimonthly journal in 1975, and has appeared monthly since 1992. The American Society of Pharmacognosy began to co-publish the journal with the American Chemical Society in 1996. In 2008, the journal was hijacked by a low-quality open access journal using the same title. , this counterfeit journal was still active. Abstracting and indexing The journal is abstracted a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dephosphorylation
In biochemistry, dephosphorylation is the removal of a phosphate (PO43−) group from an organic compound by hydrolysis. It is a reversible post-translational modification. Dephosphorylation and its counterpart, phosphorylation, activate and deactivate enzymes by detaching or attaching phosphoric esters and anhydrides. A notable occurrence of dephosphorylation is the conversion of ATP to ADP and inorganic phosphate. Dephosphorylation employs a type of hydrolytic enzyme, or hydrolase, which cleaves ester bonds. The prominent hydrolase subclass used in dephosphorylation is phosphatase, which removes phosphate groups by hydrolysing phosphoric acid monoesters into a phosphate ion and a molecule with a free hydroxyl (-OH) group. The reversible phosphorylation-dephosphorylation reaction occurs in every physiological process, making proper function of protein phosphatases necessary for organism viability. Because protein dephosphorylation is a key process involved in cell signallin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists can be activated by either endogenous agonists (such as |
5-HT2A
The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations. 5-HT is short for 5-hydroxy-tryptamine or serotonin. This is the main excitatory receptor subtype among the GPCRs for serotonin, although 5-HT2A may also have an inhibitory effect on certain areas such as the visual cortex and the orbitofrontal cortex. This receptor was first noted for its importance as a target of serotonergic psychedelic drugs such as LSD and psilocybin mushrooms. Later it came back to prominence because it was also found to be mediating, at least partly, the action of many antipsychotic drugs, especially the atypical ones. Downregulation of post-synaptic 5-HT2A receptor is an adaptive process provoked by chronic administration of selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics. Suicidal and otherwise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psilocin
Psilocin (also known as 4-HO-DMT, 4-hydroxy DMT, psilocine, psilocyn, or psilotsin) is a substituted tryptamine alkaloid and a serotonergic psychedelic substance. It is present in most psychedelic mushrooms together with its phosphorylated counterpart psilocybin. Psilocin is a Schedule I drug under the Convention on Psychotropic Substances. Acting on the 5-HT2A receptors, psilocin modulates the production and reuptake of serotonin. The mind-altering effects of psilocin are highly variable and subjective and resemble those of LSD and DMT. Chemistry Psilocin and its phosphorylated cousin, psilocybin, were first isolated and named in 1958 by Swiss chemist Albert Hofmann. Hofmann obtained the chemicals from laboratory-grown specimens of the entheogenic mushroom ''Psilocybe mexicana''. Hofmann also succeeded in finding synthetic routes to these chemicals. Psilocin can be obtained by dephosphorylation of natural psilocybin under strongly acidic or under alkaline conditions (hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptamine Alkaloids
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |