|
Nonaflate
Nonaflate, , is the common name given to nonafluorobutanesulfonates, the salts or esters of perfluorobutanesulfonic acid. Its uses are similar to those of triflate. It is a good leaving group. It is a substitute for more toxic long-chain PFAS Per- and polyfluoroalkyl substances (PFASs) are synthetic organofluorine chemical compounds that have multiple fluorine atoms attached to an alkyl chain. An early definition, from 2011, required that they contain at least one perfluoroalkyl mo ... chemicals. References Leaving groups Anions Perfluorosulfonic acids {{chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonaflate Anion
Nonaflate, , is the common name given to nonafluorobutanesulfonates, the salts or esters of perfluorobutanesulfonic acid. Its uses are similar to those of triflate. It is a good leaving group. It is a substitute for more toxic long-chain PFAS Per- and polyfluoroalkyl substances (PFASs) are synthetic organofluorine chemical compounds that have multiple fluorine atoms attached to an alkyl chain. An early definition, from 2011, required that they contain at least one perfluoroalkyl mo ... chemicals. References Leaving groups Anions Perfluorosulfonic acids {{chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorobutanesulfonic Acid
Perfluorobutanesulfonic acid (PFBS) is a chemical compound having a four-carbon fluorocarbon chain and a sulfonic acid functional group. It is stable and unreactive because of the strength of carbon–fluorine bonds. It can occur in the form of a colorless liquid or a corrosive solid. Its conjugate base is perfluorobutanesulfonate (also called nonaflate) which functions as the hydrophobe in fluorosurfactants. Since June 2003, 3M has used PFBS as a replacement for the persistent, toxic, and bioaccumulative compound perfluorooctanesulfonic acid (PFOS) in its Scotchgard stain repellents. 3M markets surfactant with PFBS in two fluorosurfactants. Safety PFBS has a half-life of a little over one month in humans, much shorter than PFOS with 5.4 years. PFBS is persistent in the environment. Studies have not yet been specifically conducted to determine safety in humans. The ECHA decision adding PFBS and its salts to the REACH Regulation Candidate List of Substances of Very High Co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
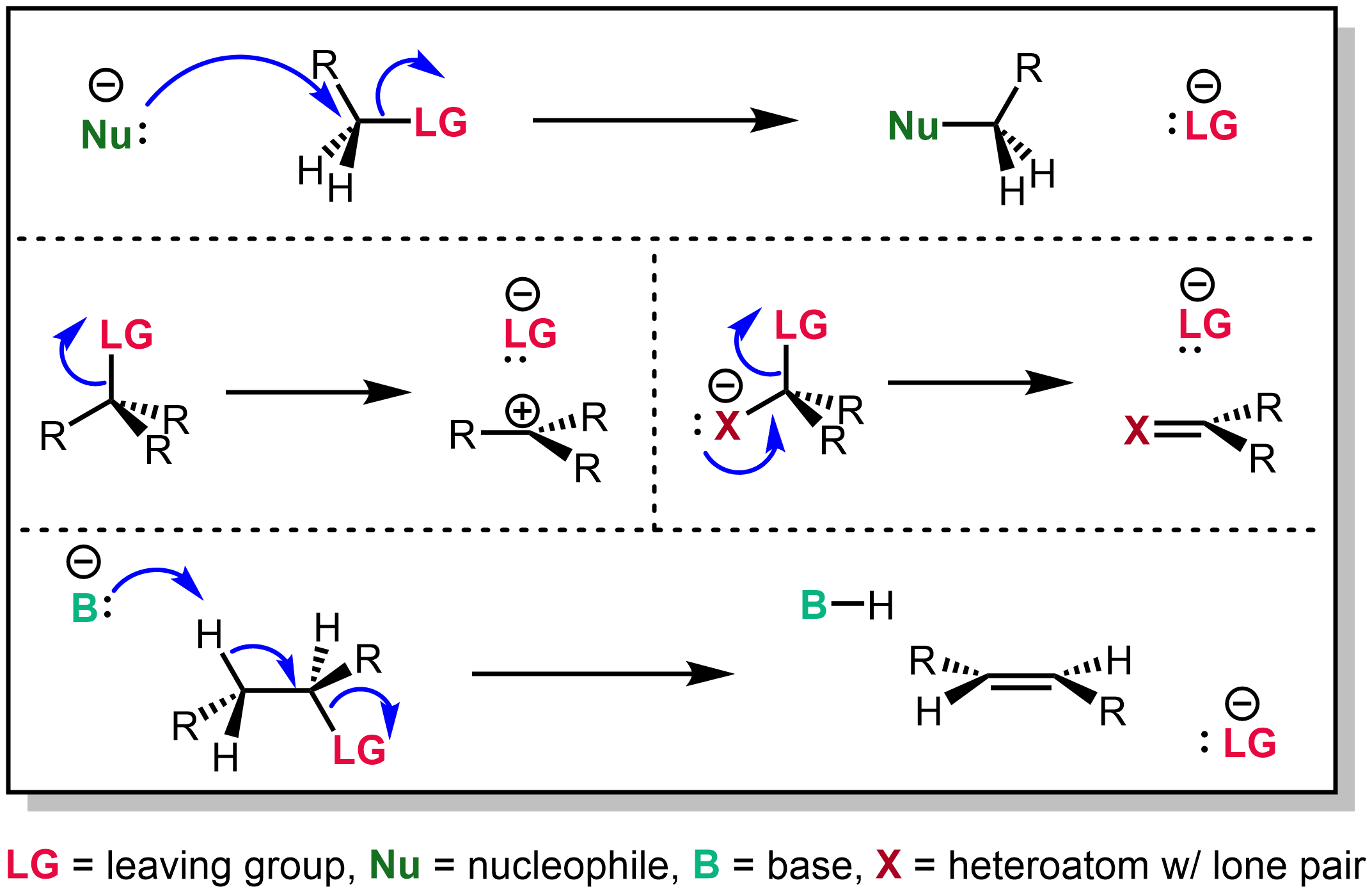
Leaving Group
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term '' nucleofuge''. In this context, leaving groups are generally anions or neutral species, departing from a neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known. A species' ability to serve as a leaving group depends on its ability to stabilize the additional electron density that results from bond heterolysis. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters such as tosylate (TsO−), while water (H2O), alcohols (HOR), and amines (R3N) are common neutr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triflate
In organic chemistry, triflate (systematic name: trifluoromethanesulfonate), is a functional group with the formula and structure . The triflate group is often represented by , as opposed to −Tf, which is the triflyl group, . For example, ''n''-butyl triflate can be written as . The corresponding triflate anion, , is an extremely stable polyatomic ion; this comes from the fact that triflic acid () is a superacid; i.e. it is more acidic than pure sulfuric acid, already one of the strongest acids known. Applications A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution, Suzuki couplings and Heck reactions. Since alkyl triflates are extremely reactive in SN2 reactions, they must be stored in conditions free of nucleophiles (such as water). The anion owes its stability to resonance stabilization which causes the negative charge to be spread symmetrically over the three oxygen atoms. An additional stabilization is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorinated Alkylated Substances
Per- and polyfluoroalkyl substances (PFASs) are synthetic organofluorine chemical compounds that have multiple fluorine atoms attached to an alkyl chain. An early definition, from 2011, required that they contain at least one perfluoroalkyl moiety, –CnF2n+1–. More recently (2021) the Organisation for Economic Co-operation and Development (OECD) expanded the definition, stating that "PFASs are defined as fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/I atom attached to it), i.e. with a few noted exceptions, any chemical with at least a perfluorinated methyl group (–CF3) or a perfluorinated methylene group (–CF2–) is a PFAS." According to the OECD, at least 4,730 distinct PFASs are known with at least three perfluorinated carbon atoms. A United States Environmental Protection Agency (EPA) toxicity database, DSSTox, lists 14,735 PFASs, while PubChem lists approximately 6 million. A subgroup, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaving Groups
Leaving or Leavin' may refer to: Film, theatre and television * ''Leaving'' (TV series), a 1984-1985 UK series featuring Keith Barron and Susan Hampshire * ''Leaving'' (1997 film), a Japanese film starring Kotomi Kyono * ''Leaving'' (2009 film), a French film by Catherine Corsini * ''Leaving'' (2011 film), a Czech film directed by Václav Havel and based on his play (see next) * ''Leaving'' (play), a 2007 play by Václav Havel * ''Leaving'' (TV series), a 2012 UK series featuring Linzey Cocker Music * ''Leaving'' (album), a 1976 album by Richard Beirach and Jeremy Steig * ''Leavin (album), a 2006 album by Natalie Cole * "Leaving" (Pet Shop Boys song), 2012 * ''Leaving'' (EP), a 2013 EP by Skrillex whose title track is "Leaving" * "Leavin' " (Jesse McCartney song), 2008 * "Leavin' " (Tony! Toni! Toné! song), 1994 * "Leaving", a song by The Starting Line from '' With Hopes of Starting Over...'' * "Leaving", a song by Westlife from ''Where We Are'' See also * * Leav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |