|
Nesosilicate
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, silica (silicon dioxide, ) is usually considered a silicate mineral. Silica is found in nature as the mineral quartz, and its polymorphism (materials science), polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this carbonate–silicate cycle, geologic cycle. For example, a type of plankton known as diatoms construct their exoskeletons ("frustules") from silica extracted from seawater. The frustules of dead diatoms are a major constituent of deep ocean sediment, and of diatomaceous e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nesosilicate
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, silica (silicon dioxide, ) is usually considered a silicate mineral. Silica is found in nature as the mineral quartz, and its polymorphism (materials science), polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this carbonate–silicate cycle, geologic cycle. For example, a type of plankton known as diatoms construct their exoskeletons ("frustules") from silica extracted from seawater. The frustules of dead diatoms are a major constituent of deep ocean sediment, and of diatomaceous e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Garnet
Garnets () are a group of silicate minerals that have been used since the Bronze Age as gemstones and abrasives. All species of garnets possess similar physical properties and crystal forms, but differ in chemical composition. The different species are pyrope, almandine, spessartine, grossular (varieties of which are hessonite or cinnamon-stone and tsavorite), uvarovite and andradite. The garnets make up two solid solution series: pyrope-almandine-spessartine (pyralspite), with the composition range ; and uvarovite-grossular-andradite (ugrandite), with the composition range . Etymology The word ''garnet'' comes from the 14th-century Middle English word ''gernet'', meaning 'dark red'. It is borrowed from Old French ''grenate'' from Latin ''granatus,'' from ''granum'' ('grain, seed'). This is possibly a reference to ''mela granatum'' or even ''pomum granatum'' ('pomegranate', ''Punica granatum''), a plant whose fruits contain abundant and vivid red seed covers ( arils), whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olivine
The mineral olivine () is a magnesium iron silicate with the chemical formula . It is a type of nesosilicate or orthosilicate. The primary component of the Earth's upper mantle, it is a common mineral in Earth's subsurface, but weathers quickly on the surface. For this reason, olivine has been proposed as a good candidate for accelerated weathering to sequester carbon dioxide from the Earth's oceans and atmosphere, as part of climate change mitigation. Olivine also has many other historical uses, such as the gemstone peridot (or chrysolite), as well as industrial applications like metalworking processes. The ratio of magnesium to iron varies between the two endmembers of the solid solution series: forsterite (Mg-endmember: ) and fayalite (Fe-endmember: ). Compositions of olivine are commonly expressed as molar percentages of forsterite (Fo) and fayalite (Fa) (''e.g.'', Fo70Fa30). Forsterite's melting temperature is unusually high at atmospheric pressure, almost , while ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plankton
Plankton are the diverse collection of organisms found in Hydrosphere, water (or atmosphere, air) that are unable to propel themselves against a Ocean current, current (or wind). The individual organisms constituting plankton are called plankters. In the ocean, they provide a crucial source of food to many small and large aquatic organisms, such as bivalves, fish and whales. Marine plankton include bacteria, archaea, algae, protozoa and drifting or floating animals that inhabit the saltwater of oceans and the brackish waters of estuaries. Freshwater plankton are similar to marine plankton, but are found in the freshwaters of lakes and rivers. Plankton are usually thought of as inhabiting water, but there are also airborne versions, the aeroplankton, that live part of their lives drifting in the atmosphere. These include plant spores, pollen and wind-scattered seeds, as well as microorganisms swept into the air from terrestrial dust storms and oceanic plankton swept into the air ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminum
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it is polarizing, and bonds aluminium forms tend towards covalency. The strong affinity towards ox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthoclase
Orthoclase, or orthoclase feldspar (endmember formula K Al Si3 O8), is an important tectosilicate mineral which forms igneous rock. The name is from the Ancient Greek for "straight fracture," because its two cleavage planes are at right angles to each other. It is a type of potassium feldspar, also known as K-feldspar. The gem known as moonstone (see below) is largely composed of orthoclase. Formation and subtypes Orthoclase is a common constituent of most granites and other felsic igneous rocks and often forms huge crystals and masses in pegmatite. Typically, the pure potassium endmember of orthoclase forms a solid solution with albite, the sodium endmember (NaAlSi3O8), of plagioclase. While slowly cooling within the earth, sodium-rich albite lamellae form by exsolution, enriching the remaining orthoclase with potassium. The resulting intergrowth of the two feldspars is called perthite. The higher-temperature polymorph of KAlSi3O8 is sanidine. Sanidine is common in rapidly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diatomaceous Earth
Diatomaceous earth (), diatomite (), or kieselgur/kieselguhr is a naturally occurring, soft, siliceous sedimentary rock that can be crumbled into a fine white to off-white powder. It has a particle size ranging from more than 3 μm to less than 1 mm, but typically 10 to 200 μm. Depending on the granularity, this powder can have an abrasive feel, similar to pumice powder, and has a low density as a result of its high porosity. The typical chemical composition of oven-dried diatomaceous earth is 80–90% silica, with 2–4% alumina (attributed mostly to clay minerals), and 0.5–2% iron oxide. Diatomaceous earth consists of the fossilized remains of diatoms, a type of hard-shelled microalgae. It is used as a filtration aid, mild abrasive in products including metal polishes and toothpaste, mechanical insecticide, absorbent for liquids, matting agent for coatings, reinforcing filler in plastics and rubber, anti-block in plastic films, porous support for c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sediment
Sediment is a naturally occurring material that is broken down by processes of weathering and erosion, and is subsequently transported by the action of wind, water, or ice or by the force of gravity acting on the particles. For example, sand and silt can be carried in suspension in river water and on reaching the sea bed deposited by sedimentation; if buried, they may eventually become sandstone and siltstone (sedimentary rocks) through lithification. Sediments are most often transported by water (fluvial processes), but also wind (aeolian processes) and glaciers. Beach sands and river channel deposits are examples of fluvial transport and deposition, though sediment also often settles out of slow-moving or standing water in lakes and oceans. Desert sand dunes and loess are examples of aeolian transport and deposition. Glacial moraine deposits and till are ice-transported sediments. Classification Sediment can be classified based on its grain size, grain shape, and c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
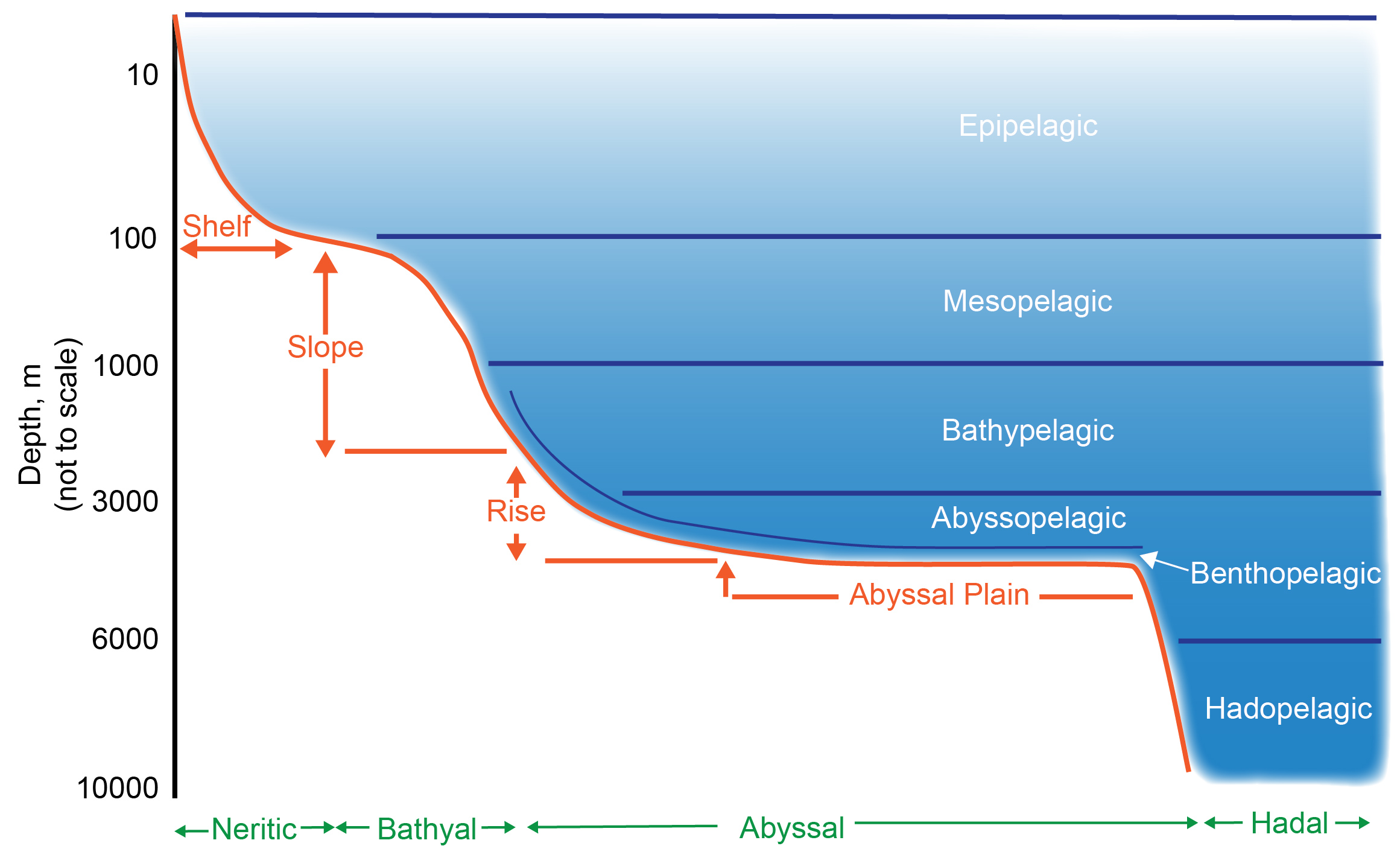
Deep Ocean
The deep sea is broadly defined as the ocean depth where light begins to fade, at an approximate depth of 200 metres (656 feet) or the point of transition from continental shelves to continental slopes. Conditions within the deep sea are a combination of low temperatures, darkness and high pressure The deep sea is considered the least explored Earth biome, with the extreme conditions making the environment difficult to access and explore. Organisms living within the deep sea have a variety of adaptations to survive in these conditions. Organisms can survive in the deep sea through a number of feeding methods including scavenging, predation and filtration, with a number of organisms surviving by feeding on marine snow. Marine snow is organic material that has fallen from upper waters into the deep sea. In 1960, the bathyscaphe ''Trieste'' descended to the bottom of the Mariana Trench near Guam, at , the deepest known spot in any ocean. If Mount Everest () were submerged there, it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |