|
Nuclear Detonation Detection System
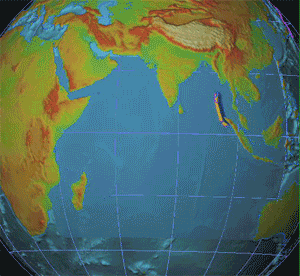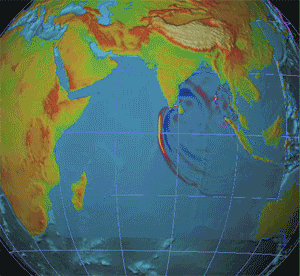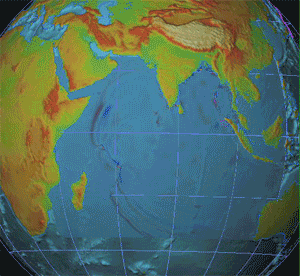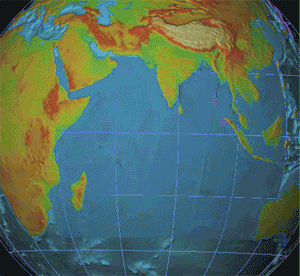
A nuclear detonation detection system (NDDS) is a device or a series of devices that are able to indicate, and pinpoint a nuclear explosion has occurred as well as the direction of the explosion. The main purpose of these devices or systems was to verify compliance of countries that signed nuclear Treaty, treaties such as the Partial Nuclear Test Ban Treaty, Partial Test Ban treaty of 1963 (PTBT) and the Treaty of Tlatelolco. There are many different ways to Detection, detect a nuclear detonation, these include Seismic wave, seismic, Hydroacoustics, hydroacoustic, and infrasound detection, Airborne particulate radioactivity monitoring, air sampling, and satellites. They have their own weaknesses and strengths, as well as different utilities. Each has been used separately, but at present the best results occur when data is used in tandem, since the energy caused by an explosion will transfer over to different mediums. Seismic Seismic networks are one of the possibilities of detonat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Explosion
A nuclear explosion is an explosion that occurs as a result of the rapid release of energy from a high-speed nuclear reaction. The driving reaction may be nuclear fission or nuclear fusion or a multi-stage cascading combination of the two, though to date all fusion-based weapons have used a fission device to initiate fusion, and a pure fusion weapon remains a hypothetical device. Nuclear explosions are used in nuclear weapons and nuclear testing. Atmospheric nuclear explosions are associated with mushroom clouds, although mushroom clouds can occur with large chemical explosions. It is possible to have an air-burst nuclear explosion without those clouds. Nuclear explosions produce radiation and radioactive debris that is harmful to humans and can cause moderate to severe skin burns, eye damage, radiation sickness, radiation-induced cancer and possible death depending on how far from the blast radius a person is. Nuclear explosions can also have detrimental effects on the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seismology
Seismology (; from Ancient Greek σεισμός (''seismós'') meaning "earthquake" and -λογία (''-logía'') meaning "study of") is the scientific study of earthquakes and the propagation of elastic waves through the Earth or through other planet-like bodies. It also includes studies of earthquake environmental effects such as tsunamis as well as diverse seismic sources such as volcanic, tectonic, glacial, fluvial, oceanic, atmospheric, and artificial processes such as explosions. A related field that uses geology to infer information regarding past earthquakes is paleoseismology. A recording of Earth motion as a function of time is called a seismogram. A seismologist is a scientist who does research in seismology. History Scholarly interest in earthquakes can be traced back to antiquity. Early speculations on the natural causes of earthquakes were included in the writings of Thales of Miletus (c. 585 BCE), Anaximenes of Miletus (c. 550 BCE), Aristotle (c. 340 BCE), and Zha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized. Xenon is used in flash lamps and arc lamps, and as a general anesthetic. The first excimer laser design used a xenon dimer molecule (Xe2) as the lasing medium, and the earliest laser designs used xenon flash lamps as pumps. Xenon is also used to search for hypothetical weakly interacting massive particles and as a propellant for ion thrusters in spacecraft. Naturally occurring xenon consists of seven stable isotopes and two long-lived radioactive isotopes. More than 40 unstable xenon isotopes undergo radioactive decay, and the isotope ratios of xenon are an important tool for studying the early history of the Solar System. Radioactive xenon-135 is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of the common isotope hydrogen-1 (''protium'') contains one proton and zero neutrons, and that of hydrogen-2 (''deuterium'') contains one proton and one neutron. Naturally occurring tritium is extremely rare on Earth. The atmosphere has only trace amounts, formed by the interaction of its gases with cosmic rays. It can be produced artificially by irradiating lithium metal or lithium-bearing ceramic pebbles in a nuclear reactor and is a low-abundance byproduct in normal operations of nuclear reactors. Tritium is used as the energy source in radioluminescent lights for watches, gun sights, numerous instruments and tools, and even novelty items such as self-illuminating key chains. It is used in a medical and scientific setting as a radioacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plutonium-239
Plutonium-239 (239Pu or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in thermal spectrum nuclear reactors, along with uranium-235 and uranium-233. Plutonium-239 has a half-life of 24,110 years. Nuclear properties The nuclear properties of plutonium-239, as well as the ability to produce large amounts of nearly pure 239Pu more cheaply than highly enriched weapons-grade uranium-235, led to its use in nuclear weapons and nuclear power plants. The fissioning of an atom of uranium-235 in the reactor of a nuclear power plant produces two to three neutrons, and these neutrons can be absorbed by uranium-238 to produce plutonium-239 and other isotopes. Plutonium-239 can also absorb neutrons and fission along with the uranium-235 in a reactor. Of all the common nuclear fuels ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium-90
Strontium-90 () is a radioactive isotope of strontium produced by nuclear fission, with a half-life of 28.8 years. It undergoes β− decay into yttrium-90, with a decay energy of 0.546 MeV. Strontium-90 has applications in medicine and industry and is an isotope of concern in fallout from nuclear weapons, nuclear weapons testing, and nuclear accidents. Radioactivity Naturally occurring strontium is nonradioactive and nontoxic at levels normally found in the environment, but 90Sr is a radiation hazard. 90Sr undergoes β− decay with a half-life of 28.79 years and a decay energy of 0.546 MeV distributed to an electron, an antineutrino, and the yttrium isotope 90Y, which in turn undergoes β− decay with a half-life of 64 hours and a decay energy of 2.28 MeV distributed to an electron, an antineutrino, and 90Zr (zirconium), which is stable. Note that 90Sr/Y is almost a pure beta particle source; the gamma photon emission from the decay of 90Y is so infrequent that it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Krypton-85
Krypton-85 (85Kr) is a radioisotope of krypton. Krypton-85 has a half-life of 10.756 years and a maximum decay energy of 687 keV. It decays into stable rubidium-85. Its most common decay (99.57%) is by beta particle emission with maximum energy of 687 keV and an average energy of 251 keV. The second most common decay (0.43%) is by beta particle emission (maximum energy of 173 keV) followed by gamma ray emission (energy of 514 keV). Other decay modes have very small probabilities and emit less energetic gamma rays. Krypton-85 is mostly synthetic, though it is produced naturally in trace quantities by cosmic ray spallation. In terms of radiotoxicity, 440 Bq of 85Kr is equivalent to 1 Bq of radon-222, without considering the rest of the radon decay chain. Presence in Earth atmosphere Natural production Krypton-85 is produced in small quantities by the interaction of cosmic rays with stable krypton-84 in the atmosphere. Natural sources maintain an equilibrium inventory of about ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium-137
Caesium-137 (), cesium-137 (US), or radiocaesium, is a radioactive isotope of caesium that is formed as one of the more common fission products by the nuclear fission of uranium-235 and other fissionable isotopes in nuclear reactors and nuclear weapons. Trace quantities also originate from spontaneous fission of uranium-238. It is among the most problematic of the short-to-medium-lifetime fission products. Caesium-137 has a relatively low boiling point of and is volatilized easily when released suddenly at high temperature, as in the case of the Chernobyl nuclear accident and with atomic explosions, and can travel very long distances in the air. After being deposited onto the soil as radioactive fallout, it moves and spreads easily in the environment because of the high water solubility of caesium's most common chemical compounds, which are salts. Caesium-137 was discovered by Glenn T. Seaborg and Margaret Melhase. Decay Caesium-137 has a half-life of about 30.05 years. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine-131
Iodine-131 (131I, I-131) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission (by weight). See fission product yield for a comparison with other radioactive fission products. 131I is also a major fission product of uranium-233, produced from thorium. Due to its mode of be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Americium-241
Americium-241 (, Am-241) is an isotope of americium. Like all isotopes of americium, it is radioactive, with a half-life of . is the most common isotope of americium as well as the most prevalent isotope of americium in nuclear waste. It is commonly found in ionization type smoke detectors and is a potential fuel for long-lifetime radioisotope thermoelectric generators (RTGs). Its common parent nuclides are β− from , EC from , and α from . is fissile and the critical mass of a bare sphere is and a sphere diameter of . Americium-241 has a specific activity of . It is commonly found in the form of americium-241 dioxide (). This isotope also has one meta state, , with an excitation energy of and a half-life of . The presence of americium-241 in plutonium is determined by the original concentration of plutonium-241 and the sample age. Because of the low penetration of alpha radiation, americium-241 only poses a health risk when ingested or inhaled. Older samples of plutoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radionuclides
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive Isotopes
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |