|
Niobium(V) Bromide
Niobium(V) bromide is the inorganic compound with the formula Nb2Br10. Its name comes from the compound's empirical formula, NbBr5. It is a diamagnetic, orange solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, which means that two NbBr5 units are joined by a pair of bromide bridges. There is no bond between the Nb centres. Niobium(V) chloride, niobium(V) iodide, tantalum(V) chloride, tantalum(V) bromide, and tantalum(V) iodide all share this structural motif. Synthesis Niobium(V) bromide can be prepared by the reaction of bromine with niobium metal at 230-250 °C in a tube furnace. It can also be produced from the more accessible oxide by metathesis using aluminium tribromide Aluminium bromide is any chemical compound with the empirical formula AlBrx. Aluminium tribromide is the most common form of aluminium bromide. It is a colorless, sublimable hygroscopic solid; hence old samples tend to be hydrated, mostly as al ...: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Empirical Formula
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the empirical formula of disulfur dioxide, S2O2. Thus, sulfur monoxide and disulfur dioxide, both compounds of sulfur and oxygen, have the same empirical formula. However, their molecular formulas, which express the number of atoms in each molecule of a chemical compound, are not the same. An empirical formula makes no mention of the arrangement or number of atoms. It is standard for many ionic compounds, like calcium chloride (CaCl2), and for macromolecules, such as silicon dioxide (SiO2). The molecular formula, on the other hand, shows the number of each type of atom in a molecule. The structural formula shows the arrangement of the molecule. It is also possible for different types of compounds to have equal empirical formulas. Sampl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
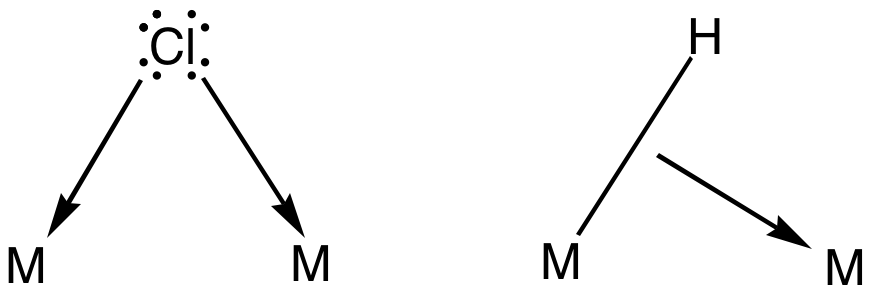
Bridging Ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals. In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ, with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands. List of bridging ligands Virtually all ligands are known to bridge, with the exception of amines and ammonia. Common bridging ligands include most of the common anions. Many simple organic ligands form str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niobium(V) Chloride
Niobium(V) chloride, also known as niobium pentachloride, is a yellow crystalline solid. It hydrolyzes in air, and samples are often contaminated with small amounts of NbOCl3. It is often used as a precursor to other compounds of niobium. NbCl5 may be purified by sublimation. Structure and properties Niobium(V) chloride forms chloro-bridged dimers in the solid state (''see'' figure). Each niobium centre is six-coordinate, but the octahedral coordination is significantly distorted. The equatorial niobium–chlorine bond lengths are 225 pm (terminal) and 256 pm (bridging), whilst the axial niobium-chlorine bonds are 229.2 pm and are deflected inwards to form an angle of 83.7° with the equatorial plane of the molecule. The Nb–Cl–Nb angle at the bridge is 101.3°. The Nb–Nb distance is 398.8 pm, too long for any metal-metal interaction. NbBr5, TaCl5 and TaBr5 are isostructural with NbCl5, but NbI5 and TaI5 have different structures. Preparation Industrial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niobium(V) Iodide
Niobium(V) iodide is the inorganic compound with the formula Nb2I10. Its name comes from the compound's empirical formula, NbI5. It is a diamagnetic, yellow solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, which means that two NbI5 units are joined by a pair of iodide bridges. There is no bond between the Nb centres. Niobium(V) chloride, niobium(V) bromide, tantalum(V) chloride, tantalum(V) bromide, and tantalum(V) iodide Tantalum(V) iodide is the inorganic compound with the formula Ta2I10. Its name comes from the compound's empirical formula, TaI5. It is a diamagnetic, black solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, ..., all share this structural motif. Synthesis and structure Niobium pentaiodide forms from the reaction of niobium with iodine: :2 Nb + 5 I2 → 2 NbI5 The method used for the preparation of tantalum(V) iodide using aluminium triiodide fails to produce pure pentai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tantalum(V) Chloride
Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxychloride (TaOCl3) and eventually tantalum pentoxide (Ta2O5); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques. Structure TaCl5 crystallizes in the monoclinic space group ''C''2/''m''. The ten chlorine atoms define a pair of octahedra that share a common edge. The tantalum atoms occupy the centres of the octahedra and are joined by two chlorine bridging ligands. The dimeric structure is retained in non-complexing solvents and to a large extent in the molten state. In the vapour state, however, TaCl5 is monomeric. This monomer adopts a trigonal bipyramidal structure, like that of PCl5. Physical Properties The solubility of tantalum pentachloride increases slightly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tantalum(V) Bromide
Tantalum(V) bromide is the inorganic compound with the formula Ta2Br10. Its name comes from the compound's empirical formula, TaBr5. It is a diamagnetic, orange solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, which means that two TaBr5 units are joined by a pair of bromide bridges. There is no bond between the Ta centres. Niobium(V) chloride, niobium(V) bromide, niobium(V) iodide, tantalum(V) chloride, and tantalum(V) iodide all share this structural motif. Preparation and handling The material is usually prepared by the reaction of bromine with tantalum metal (or tantalum carbide) at elevated temperatures in a tube furnace. The bromides of the early metals are sometimes preferred to the chlorides because of the relative ease of handling liquid bromine vs gaseous chlorine. Like other molecular halides, it is soluble in nonpolar solvents such as carbon tetrachloride (1.465 g/100 mL at 30 °C), but it reacts with some solvents. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tantalum(V) Iodide
Tantalum(V) iodide is the inorganic compound with the formula Ta2I10. Its name comes from the compound's empirical formula, TaI5. It is a diamagnetic, black solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral structure, which means that two TaI5 units are joined by a pair of iodide bridges. There is no bond between the Ta centres. Niobium(V) chloride, niobium(V) bromide, niobium(V) iodide, tantalum(V) chloride, and tantalum(V) bromide Tantalum(V) bromide is the inorganic compound with the formula Ta2Br10. Its name comes from the compound's empirical formula, TaBr5. It is a diamagnetic, orange solid that hydrolyses readily. The compound adopts an edge-shared bioctahedral struc ... all share this structural motif. Synthesis and structure Tantalum pentaiodide forms from the reaction of tantalum pentoxide with aluminium triiodide: :3 Ta2O5 + 10 AlI3 → 6 TaI5 + 5 Al2O3 References {{Iodides Iodides Tantalum compounds Metal halides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tube Furnace
A tube furnace is an electric heating device used to conduct syntheses and purifications of inorganic compounds and occasionally in organic synthesis. One possible design consists of a cylindrical cavity surrounded by heating coils that are embedded in a thermally insulating matrix. Temperature can be controlled via feedback from a thermocouple. More elaborate tube furnaces have two (or more) heating zones useful for transport experiments. Some digital temperature controllers provide an RS-232 interface, and permit the operator to program segments for uses like ramping, soaking, sintering, and more. Advanced materials in the heating elements, such as molybdenum disilicide (MoSi2) offered in certain models can now produce working temperatures up to 1800 °C. This facilitates more sophisticated applications. Common material for the reaction tubes include alumina, Pyrex, and fused quartz, or in the case of corrosive materials molybdenum or tungsten tubes can be used. The tube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Tribromide
Aluminium bromide is any chemical compound with the empirical formula AlBrx. Aluminium tribromide is the most common form of aluminium bromide. It is a colorless, sublimable hygroscopic solid; hence old samples tend to be hydrated, mostly as aluminium tribromide hexahydrate (AlBr3·6H2O). Structure The dimeric form of aluminium tribromide (Al2Br6) predominates in the solid state, in solutions in noncoordinating solvents (e.g. CS2), in the melt, and in the gas phase. Only at high temperatures do these dimers break up into monomers: : Al2Br6 → 2 AlBr3 ΔH°diss = 59 kJ/mol The species aluminium monobromide forms from the reaction of HBr with Al metal at high temperature. It disproportionates near room temperature: :6/n " lBrsub>n" → Al2Br6 + 4 Al This reaction is reversed at temperatures higher than 1000 °C. Aluminium monobromide has been crystallographically characterized in the form the tetrameric adduct Al4Br4(NEt3)4 (Et = C2H5). This species is electronical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromides
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardant materials, and cell stains. Although uncommon, chronic toxicity from bromide can result in bromism, a syndrome with multiple neurological symptoms. Bromide toxicity can also cause a type of skin eruption, see potassium bromide. The bromide ion has an ionic radius of 196 pm. Natural occurrence Bromide is present in typical seawater (35 PSU) with a concentration of around 65 mg/L, which is about 0.2% of all dissolved salts. Seafood and deep sea plants generally have higher levels than land-derived foods. Bromargyrite—natural, crystalline silver bromide—is the most common bromide mineral known but is still very rare. In addition to silver, bromine is also in minerals combined with mercury and copper. Formation and react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Halides
Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride. File:NaCl polyhedra.png, Sodium chloride crystal structure File:Uranium-hexafluoride-unit-cell-3D-balls.png, Discrete UF6 molecules File:Alpha-palladium(II)-chloride-xtal-3D-balls.png, Infinite chains of one form of palladium chloride Preparation The halogens can all react with metals to form metal halides according to the following equation: :2M + nX2 → 2MXn where M is the metal, X is the halogen, and MXn is the metal halide. In practice, this type of reaction may be very exothermic, hence impractical as a preparative technique. Additionally, many transition metals can adopt multiple oxidation states, which complicates matters. As the halogens are strong oxidizers, direct combination of the elements usua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |