|
Neutron Reflectometry
Neutron reflectometry is a neutron diffraction technique for measuring the structure of thin films, similar to the often complementary techniques of X-ray reflectivity and ellipsometry. The technique provides valuable information over a wide variety of scientific and technological applications including chemical aggregation, polymer and surfactant adsorption, structure of thin film magnetic systems, biological membranes, etc. History Neutron reflectometery emerged as a new field in the 1980s, after the discovery of giant magnetoresistance in antiferromagnetically-coupled multilayered films. Technique The technique involves shining a highly collimated beam of neutrons onto an extremely flat surface and measuring the intensity of reflected radiation as a function of angle or neutron wavelength. The exact shape of the reflectivity profile provides detailed information about the structure of the surface, including the thickness, density, and roughness of any thin films layered on th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Diffraction
Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material. A sample to be examined is placed in a beam of thermal or cold neutrons to obtain a diffraction pattern that provides information of the structure of the material. The technique is similar to X-ray diffraction but due to their different scattering properties, neutrons and X-rays provide complementary information: X-Rays are suited for superficial analysis, strong x-rays from synchrotron radiation are suited for shallow depths or thin specimens, while neutrons having high penetration depth are suited for bulk samples.Measurement of residual stress in materials using neutrons |
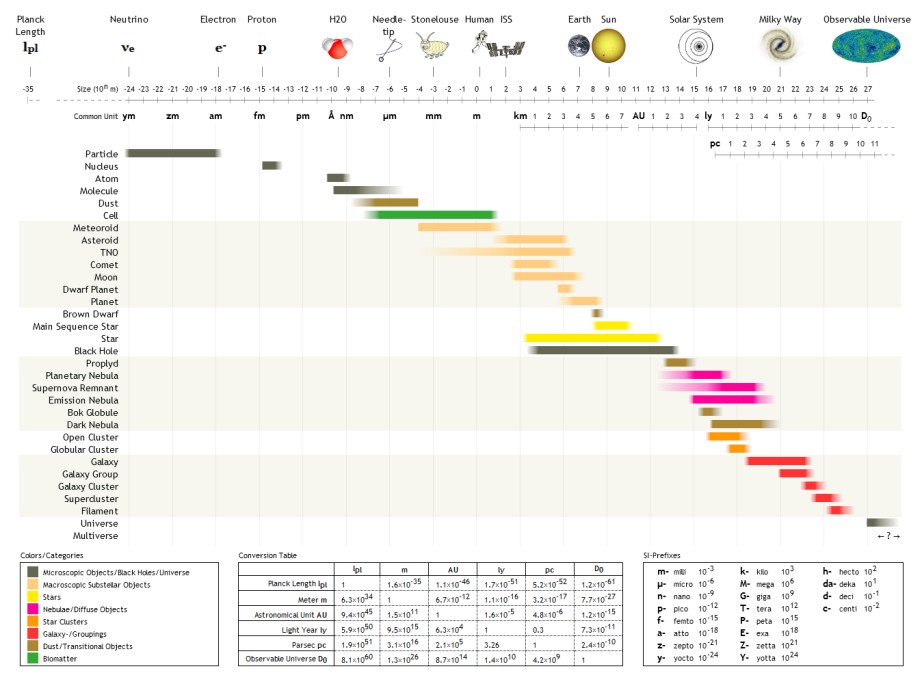
Terameter
The following are examples of orders of magnitude for different lengths. __TOC__ Overview Detailed list To help compare different orders of magnitude, the following list describes various lengths between 1.6 \times 10^ metres and 10^metres. Subatomic scale Atomic to cellular scale Cellular to human scale Human to astronomical scale Astronomical scale Less than 1 zeptometre The ' ( SI symbol: ') is a unit of length in the metric system equal to . To help compare different orders of magnitude, this section lists lengths shorter than 10−21 m (1 zm). *1.6 × 10−5 quectometres (1.6 × 10−35 metres) – the Planck length (Measures of distance shorter than this do not make physical sense, according to current theories of physics.) *1 qm – 1 quectometre, the smallest named subdivision of the metre in the SI base unit of length, one nonillionth of a metre *1 rm – 1 rontometre, a subdivision of the metre in the SI base unit of length, one octilliont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many industrially ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three isotopes occur naturally, C and C being stable, while C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity. Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of Carbon-based life, all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematically, density is defined as mass divided by volume: : \rho = \frac where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate – this quantity is more specifically called specific weight. For a pure substance the density has the same numerical value as its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium and iridium are the densest known elements at standard conditions for temperature and pressure. To simplify comparisons of density across different syst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Scattering Length
A neutron may pass by a nucleus with a probability determined by the nuclear interaction distance, or be absorbed, or undergo scattering that may be either coherent or incoherent. The interference effects in coherent scattering can be computed via the coherent scattering length of neutrons, being proportional to the amplitude of the spherical scattered waves according to Huygens–Fresnel theory. This scattering length varies by isotope (and by element as the weighted arithmetic mean over the constituent isotopes) in a way that appears random, whereas the X-ray scattering length is just the product of atomic number and Thomson scattering length, thus monotonically increasing with atomic number. The scattering length may be either positive or negative. The scattering cross-section is equal to the square of the scattering length multiplied by 4π, i.e. the area of a circle with radius twice the scattering length. In some cases, as with titanium and nickel, it is possible to mix isot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler substances by any chemical reaction. The number of protons in the nucleus is the defining property of an element, and is referred to as its atomic number (represented by the symbol ''Z'') – all atoms with the same atomic number are atoms of the same element. Almost all of the baryonic matter of the universe is composed of chemical elements (among rare exceptions are neutron stars). When different elements undergo chemical reactions, atoms are rearranged into new compounds held together by chemical bonds. Only a minority of elements, such as silver and gold, are found uncombined as relatively pure native element minerals. Nearly all other naturally occurring elements occur in the Earth as compounds or mixtures. Air is primarily a m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties. The term isotope is formed from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in 1913 in a suggestion to the British chemist Frederick Soddy. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Scattering
Neutron scattering, the irregular dispersal of free neutrons by matter, can refer to either the naturally occurring physical process itself or to the man-made experimental techniques that use the natural process for investigating materials. The natural/physical phenomenon is of elemental importance in nuclear engineering and the nuclear sciences. Regarding the experimental technique, understanding and manipulating neutron scattering is fundamental to the applications used in crystallography, physics, physical chemistry, biophysics, and materials research. Neutron scattering is practiced at research reactors and spallation neutron sources that provide neutron radiation of varying intensities. Neutron diffraction ( elastic scattering) techniques are used for analyzing structures; where inelastic neutron scattering is used in studying atomic vibrations and other excitations. Scattering of fast neutrons "Fast neutrons" (see neutron temperature) have a kinetic energy abov ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Particle Accelerator
A particle accelerator is a machine that uses electromagnetic fields to propel electric charge, charged particles to very high speeds and energies, and to contain them in well-defined particle beam, beams. Large accelerators are used for fundamental research in particle physics. The largest accelerator currently active is the Large Hadron Collider (LHC) near Geneva, Switzerland, operated by the European Organization for Nuclear Research, CERN. It is a collider accelerator, which can accelerate two beams of protons to an energy of 6.5 TeV and cause them to collide head-on, creating center-of-mass energies of 13 TeV. Other powerful accelerators are, Relativistic Heavy Ion Collider, RHIC at Brookhaven National Laboratory in New York and, formerly, the Tevatron at Fermilab, Batavia, Illinois. Accelerators are also used as synchrotron light sources for the study of condensed matter physics. Smaller particle accelerators are used in a wide variety of applications, includ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spallation
Spallation is a process in which fragments of material ( spall) are ejected from a body due to impact or stress. In the context of impact mechanics it describes ejection of material from a target during impact by a projectile. In planetary physics, spallation describes meteoritic impacts on a planetary surface and the effects of stellar winds and cosmic rays on planetary atmospheres and surfaces. In the context of mining or geology, spallation can refer to pieces of rock breaking off a rock face due to the internal stresses in the rock; it commonly occurs on mine shaft walls. In the context of anthropology, spallation is a process used to make stone tools such as arrowheads by knapping. In nuclear physics, spallation is the process in which a heavy nucleus emits numerous nucleons as a result of being hit by a high-energy particle, thus greatly reducing its atomic weight. In industrial processes and bioprocessing the loss of tubing material due to the repeated flexing of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)

%2C_black_and_white.png)
