|
Microscopic Reversibility
The principle of microscopic reversibility in physics and chemistry is twofold: * First, it states that the microscopic detailed dynamics of particles and fields is time-reversible because the microscopic equations of motion are symmetric with respect to inversion in time (T-symmetry); * Second, it relates to the statistical description of the kinetics of macroscopic or mesoscopic systems as an ensemble of elementary processes: collisions, elementary transitions or reactions. For these processes, the consequence of the microscopic T-symmetry is: ''Corresponding to every individual process there is a reverse process, and in a state of equilibrium the average rate of every process is equal to the average rate of its reverse process.'' History of microscopic reversibility The idea of microscopic reversibility was born together with physical kinetics. In 1872, Ludwig Boltzmann represented kinetics of gases as statistical ensemble of elementary collisions.Boltzmann, L. (1964), Lectures on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physics
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which relates to the order of nature, or, in other words, to the regular succession of events." Physics is one of the most fundamental scientific disciplines, with its main goal being to understand how the universe behaves. "Physics is one of the most fundamental of the sciences. Scientists of all disciplines use the ideas of physics, including chemists who study the structure of molecules, paleontologists who try to reconstruct how dinosaurs walked, and climatologists who study how human activities affect the atmosphere and oceans. Physics is also the foundation of all engineering and technology. No engineer could design a flat-screen TV, an interplanetary spacecraft, or even a better mousetrap without first understanding the basic laws of physic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
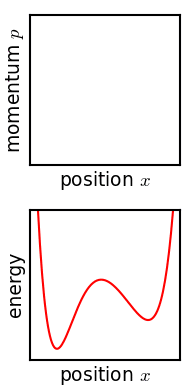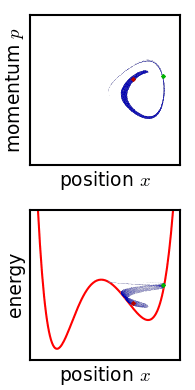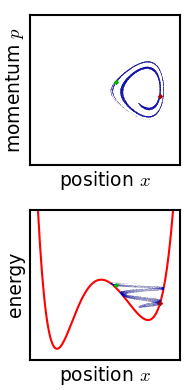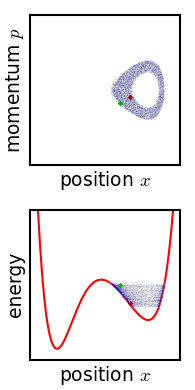
Configuration Space (physics)
In classical mechanics, the parameters that define the configuration of a system are called ''generalized coordinates,'' and the space defined by these coordinates is called the configuration space of the physical system. It is often the case that these parameters satisfy mathematical constraints, such that the set of actual configurations of the system is a manifold in the space of generalized coordinates. This manifold is called the configuration manifold of the system. Notice that this is a notion of "unrestricted" configuration space, i.e. in which different point particles may occupy the same position. In mathematics, in particular in topology, a notion of "restricted" configuration space is mostly used, in which the diagonals, representing "colliding" particles, are removed. Example: a particle in 3D space The position of a single particle moving in ordinary Euclidean 3-space is defined by the vector q=(x,y,z), and therefore its ''configuration space'' is Q=\mathbb^3. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Detailed Balance
The principle of detailed balance can be used in kinetic systems which are decomposed into elementary processes (collisions, or steps, or elementary reactions). It states that at equilibrium, each elementary process is in equilibrium with its reverse process. History The principle of detailed balance was explicitly introduced for collisions by Ludwig Boltzmann. In 1872, he proved his H-theorem using this principle.Boltzmann, L. (1964), Lectures on gas theory, Berkeley, CA, USA: U. of California Press. The arguments in favor of this property are founded upon microscopic reversibility. Tolman, R. C. (1938). ''The Principles of Statistical Mechanics''. Oxford University Press, London, UK. Five years before Boltzmann, James Clerk Maxwell used the principle of detailed balance for gas kinetics with the reference to the principle of sufficient reason. He compared the idea of detailed balance with other types of balancing (like cyclic balance) and found that "Now it is impossible to as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Law Of Mass Action
In chemistry, the law of mass action is the proposition that the rate of the chemical reaction is directly proportional to the product of the activities or concentrations of the reactants. It explains and predicts behaviors of solutions in dynamic equilibrium. Specifically, it implies that for a chemical reaction mixture that is in equilibrium, the ratio between the concentration of reactants and products is constant. Two aspects are involved in the initial formulation of the law: 1) the equilibrium aspect, concerning the composition of a reaction mixture at equilibrium and 2) the kinetic aspect concerning the rate equations for elementary reactions. Both aspects stem from the research performed by Cato M. Guldberg and Peter Waage between 1864 and 1879 in which equilibrium constants were derived by using kinetic data and the rate equation which they had proposed. Guldberg and Waage also recognized that chemical equilibrium is a dynamic process in which rates of reaction for t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helmholtz
Hermann Ludwig Ferdinand von Helmholtz (31 August 1821 – 8 September 1894) was a German physicist and physician who made significant contributions in several scientific fields, particularly hydrodynamic stability. The Helmholtz Association, the largest German association of research institutions, is named in his honor. In the fields of physiology and psychology, Helmholtz is known for his mathematics concerning the eye, theories of vision, ideas on the visual perception of space, color vision research, the sensation of tone, perceptions of sound, and empiricism in the physiology of perception. In physics, he is known for his theories on the conservation of energy, work in electrodynamics, chemical thermodynamics, and on a mechanical foundation of thermodynamics. As a philosopher, he is known for his philosophy of science, ideas on the relation between the laws of perception and the laws of nature, the science of aesthetics, and ideas on the civilizing power of science. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thomson Effect
The thermoelectric effect is the direct conversion of temperature differences to electric voltage and vice versa via a thermocouple. A thermoelectric device creates a voltage when there is a different temperature on each side. Conversely, when a voltage is applied to it, heat is transferred from one side to the other, creating a temperature difference. At the atomic scale, an applied temperature gradient causes charge carriers in the material to diffuse from the hot side to the cold side. This effect can be used to generate electricity, measure temperature or change the temperature of objects. Because the direction of heating and cooling is affected by the applied voltage, thermoelectric devices can be used as temperature controllers. The term "thermoelectric effect" encompasses three separately identified effects: the Seebeck effect, Peltier effect, and Thomson effect. The Seebeck and Peltier effects are different manifestations of the same physical process; textbooks may re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
H-theorem
In classical statistical mechanics, the ''H''-theorem, introduced by Ludwig Boltzmann in 1872, describes the tendency to decrease in the quantity ''H'' (defined below) in a nearly-ideal gas of molecules. L. Boltzmann,Weitere Studien über das Wärmegleichgewicht unter Gasmolekülen" Sitzungsberichte Akademie der Wissenschaften 66 (1872): 275-370. English translation: As this quantity ''H'' was meant to represent the entropy of thermodynamics, the ''H''-theorem was an early demonstration of the power of statistical mechanics as it claimed to derive the second law of thermodynamics—a statement about fundamentally irreversible processes—from reversible microscopic mechanics. It is thought to prove the second law of thermodynamics, albeit under the assumption of low-entropy initial conditions. The ''H''-theorem is a natural consequence of the kinetic equation derived by Boltzmann that has come to be known as Boltzmann's equation. The ''H''-theorem has led to considerable discuss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy Production
Entropy production (or generation) is the amount of entropy which is produced in any irreversible processes such as heat and mass transfer processes including motion of bodies, heat exchange, fluid flow, substances expanding or mixing, anelastic deformation of solids, and any irreversible thermodynamic cycle, including thermal machines such as power plants, heat engines, refrigerators, heat pumps, and air conditioners. In the dual representation entropy–exergy for accounting the second law of thermodynamics it can be expressed in equivalent terms of exergy disruption. Short history Entropy is produced in irreversible processes. The importance of avoiding irreversible processes (hence reducing the entropy production) was recognized as early as 1824 by Carnot. In 1865 Rudolf Clausius expanded his previous work from 1854 on the concept of "unkompensierte Verwandlungen" (uncompensated transformations), which, in our modern nomenclature, would be called the entropy production. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann Equation
The Boltzmann equation or Boltzmann transport equation (BTE) describes the statistical behaviour of a thermodynamic system not in a state of equilibrium, devised by Ludwig Boltzmann in 1872.Encyclopaedia of Physics (2nd Edition), R. G. Lerner, G. L. Trigg, VHC publishers, 1991, ISBN (Verlagsgesellschaft) 3-527-26954-1, ISBN (VHC Inc.) 0-89573-752-3. The classic example of such a system is a fluid with temperature gradients in space causing heat to flow from hotter regions to colder ones, by the random but biased transport of the particles making up that fluid. In the modern literature the term Boltzmann equation is often used in a more general sense, referring to any kinetic equation that describes the change of a macroscopic quantity in a thermodynamic system, such as energy, charge or particle number. The equation arises not by analyzing the individual positions and momenta of each particle in the fluid but rather by considering a probability distribution for the positio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Onsager Reciprocal Relations
In thermodynamics, the Onsager reciprocal relations express the equality of certain ratios between flows and forces in thermodynamic systems out of equilibrium, but where a notion of local equilibrium exists. "Reciprocal relations" occur between different pairs of forces and flows in a variety of physical systems. For example, consider fluid systems described in terms of temperature, matter density, and pressure. In this class of systems, it is known that temperature differences lead to heat flows from the warmer to the colder parts of the system; similarly, pressure differences will lead to matter flow from high-pressure to low-pressure regions. What is remarkable is the observation that, when both pressure and temperature vary, temperature differences at constant pressure can cause matter flow (as in convection) and pressure differences at constant temperature can cause heat flow. Perhaps surprisingly, the heat flow per unit of pressure difference and the density (matter) flo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CPT Symmetry
Charge, parity, and time reversal symmetry is a fundamental symmetry of physical laws under the simultaneous transformations of charge conjugation (C), parity transformation (P), and time reversal (T). CPT is the only combination of C, P, and T that is observed to be an exact symmetry of nature at the fundamental level. The CPT theorem says that CPT symmetry holds for all physical phenomena, or more precisely, that any Lorentz invariant local quantum field theory with a Hermitian Hamiltonian must have CPT symmetry. History The CPT theorem appeared for the first time, implicitly, in the work of Julian Schwinger in 1951 to prove the connection between spin and statistics. In 1954, Gerhart Lüders and Wolfgang Pauli derived more explicit proofs, so this theorem is sometimes known as the Lüders–Pauli theorem. At about the same time, and independently, this theorem was also proved by John Stewart Bell. These proofs are based on the principle of Lorentz invariance and the princ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Weak Interaction
In nuclear physics and particle physics, the weak interaction, which is also often called the weak force or weak nuclear force, is one of the four known fundamental interactions, with the others being electromagnetism, the strong interaction, and gravitation. It is the mechanism of interaction between subatomic particles that is responsible for the radioactive decay of atoms: The weak interaction participates in nuclear fission and nuclear fusion. The theory describing its behaviour and effects is sometimes called quantum flavourdynamics (QFD); however, the term QFD is rarely used, because the weak force is better understood by Electroweak interaction, electroweak theory (EWT). The effective range of the weak force is limited to subatomic distances and is less than the diameter of a proton. Background The Standard Model of particle physics provides a uniform framework for understanding electromagnetic, weak, and strong interactions. An interaction occurs when two particles ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |