|
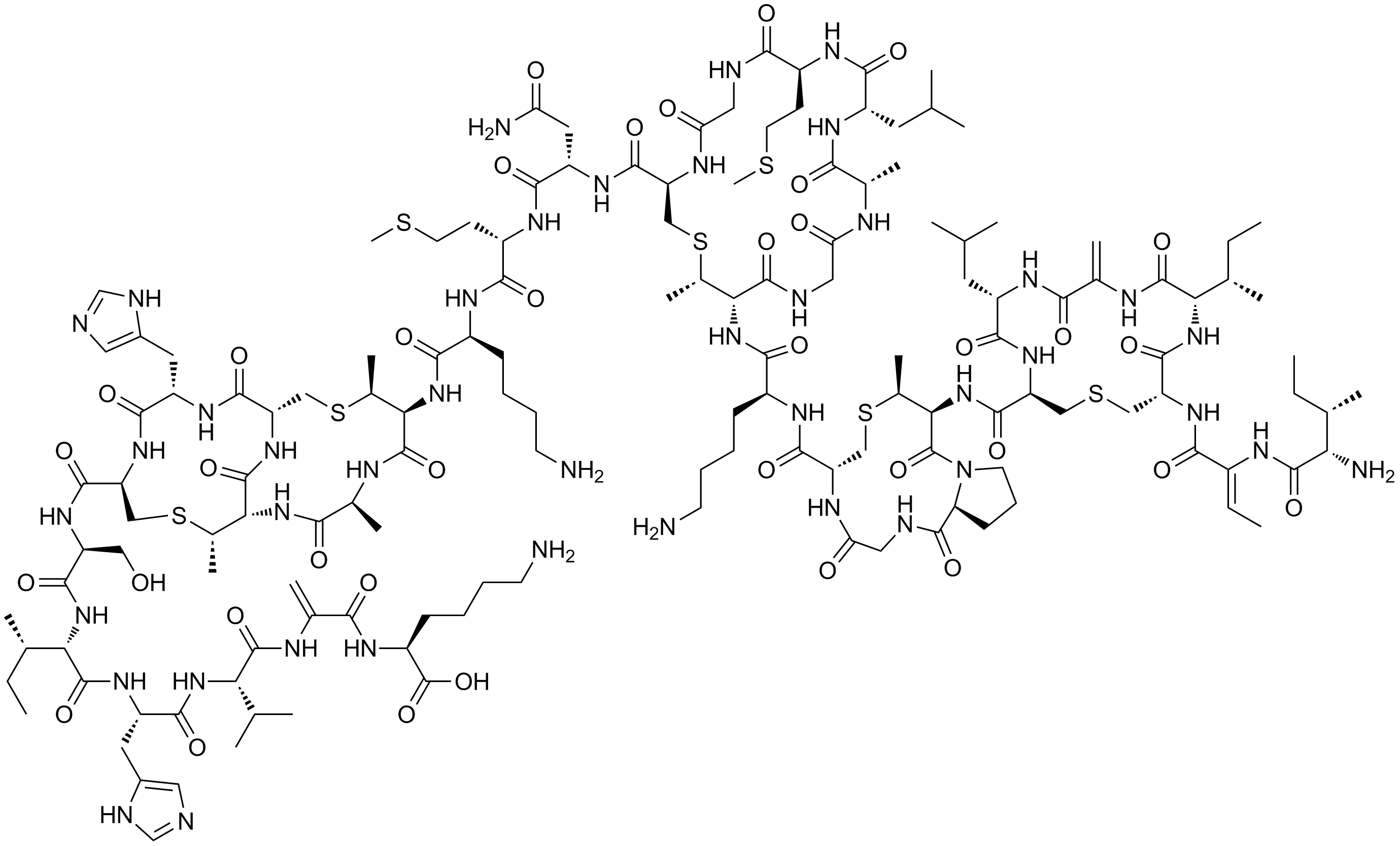
Microcystins
Microcystins—or cyanoginosins—are a class of toxins produced by certain freshwater cyanobacteria, commonly known as blue-green algae. Over 250 different microcystins have been discovered so far, of which microcystin-LR is the most common. Chemically they are cyclic heptapeptides produced through nonribosomal peptide synthases. Cyanobacteria can produce microcystins in large quantities during algal blooms which then pose a major threat to drinking and irrigation water supplies, and the environment at large. Characteristics Microcystins—or cyanoginosins—are a class of toxins produced by certain freshwater cyanobacteria; primarily ''Microcystis aeruginosa'' but also other ''Microcystis'', as well as members of the ''Planktothrix'', ''Anabaena'', ''Oscillatoria'' and ''Nostoc'' genera. Over 250 different microcystins have been discovered so far, of which microcystin-LR is the most common. Chemically they are cyclic heptapeptides produced through nonribosomal peptide synthas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microcystin-LR
Microcystin-LR (MC-LR) is a toxin produced by cyanobacteria. It is the most toxic of the microcystins. Structure Microcystins are cyclic heptapeptides. The seven amino acids that are involved in the structure of a microcystin include a unique β-amino acid ( ADDA). It contains alanine (D-ala), D-β-methyl-isoaspartate (D-β-Me-isoAsp), and glutamic acid (D-glu). Furthermore, microcystins contain two variable residues, which make the differentiation between variants of microcystins. These two variable elements are always standard L-amino acids. In microcystin-LR these are leucine and arginine. more than 250 microcystins have been identified to date,''Structural Diversity, Characterization and Toxicology of Microcystins'', doi: 10.3390/toxins11120714 representing differences in the two variable residues and some modifications in the other amino acids. These modifications include demethylation of Masp and Mdha and methylesterification of D-Glu. Different microcystins have differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microcystin-LR
Microcystin-LR (MC-LR) is a toxin produced by cyanobacteria. It is the most toxic of the microcystins. Structure Microcystins are cyclic heptapeptides. The seven amino acids that are involved in the structure of a microcystin include a unique β-amino acid ( ADDA). It contains alanine (D-ala), D-β-methyl-isoaspartate (D-β-Me-isoAsp), and glutamic acid (D-glu). Furthermore, microcystins contain two variable residues, which make the differentiation between variants of microcystins. These two variable elements are always standard L-amino acids. In microcystin-LR these are leucine and arginine. more than 250 microcystins have been identified to date,''Structural Diversity, Characterization and Toxicology of Microcystins'', doi: 10.3390/toxins11120714 representing differences in the two variable residues and some modifications in the other amino acids. These modifications include demethylation of Masp and Mdha and methylesterification of D-Glu. Different microcystins have differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
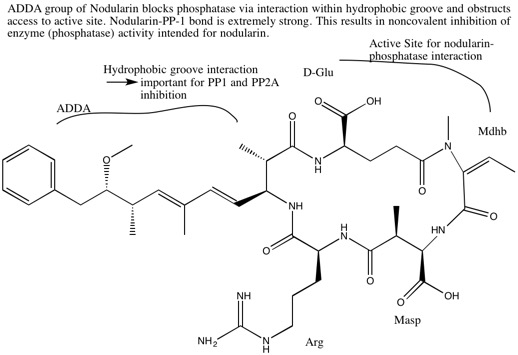
Nodularin
Nodularins are potent toxins produced by the cyanobacterium ''Nodularia spumigena'', among others. This aquatic, photosynthetic cyanobacterium forms visible colonies that present as algal blooms in brackish water bodies throughout the world. The late summer blooms of ''Nodularia spumigena'' are among the largest cyanobacterial mass occurrences in the world. Cyanobacteria are composed of many toxic substances, most notably of microcystins and nodularins: the two are not easily differentiated. A significant homology of structure and function exists between the two, and microcystins have been studied in greater detail. Because of this, facts from microcystins are often extended to nodularins. Nodularin-R is the predominant toxin variant, though 10 variants of nodularin have been discovered to date. Nodularins are cyclic nonribosomal pentapeptides and contain several unusual non-proteinogenic amino acids such as N-methyl-didehydroaminobutyric acid and the β-amino acid ADDA. These c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Planktothrix
''Planktothrix'' is a diverse genus of filamentous cyanobacteria observed to amass in algal blooms in water ecosystems across the globe. Like all Oscillatoriales, ''Planktothrix'' species have no heterocysts and no akinetes. Planktothrix are unique because they have trichomes and contain gas vacuoles unlike typical planktonic organisms. Previously, some species of the taxon were grouped within the genus ''Oscillatoria,'' but recent work has defined Planktothrix as its own genus. A tremendous body of work on ''Planktothrix'' ecology and physiology has been done by Anthony E. Walsby, and the 55.6 kb microcystin synthetase gene which gives these organisms the ability to synthesize toxins has been sequenced. ''P. agardhii'' is an example of a type species of the genus. ''P. agardhii'' and ''P. rubescens'' are commonly observed in lakes of the Northern Hemisphere where they are known producers of potent hepatotoxins called microcystins. Habitats and niches Both ''P. agardhii'' and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(all-S,all-E)-3-Amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-diene Acid
ADDA ((all-''S'',all-''E'')-3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid) is a non-proteinogenic amino acid found in toxins made by cyanobacteria. Toxins which include this amino acid include microcystins and nodularin Nodularins are potent toxins produced by the cyanobacterium '' Nodularia spumigena'', among others. This aquatic, photosynthetic cyanobacterium forms visible colonies that present as algal blooms in brackish water bodies throughout the world. The ...s. References Non-proteinogenic amino acids {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
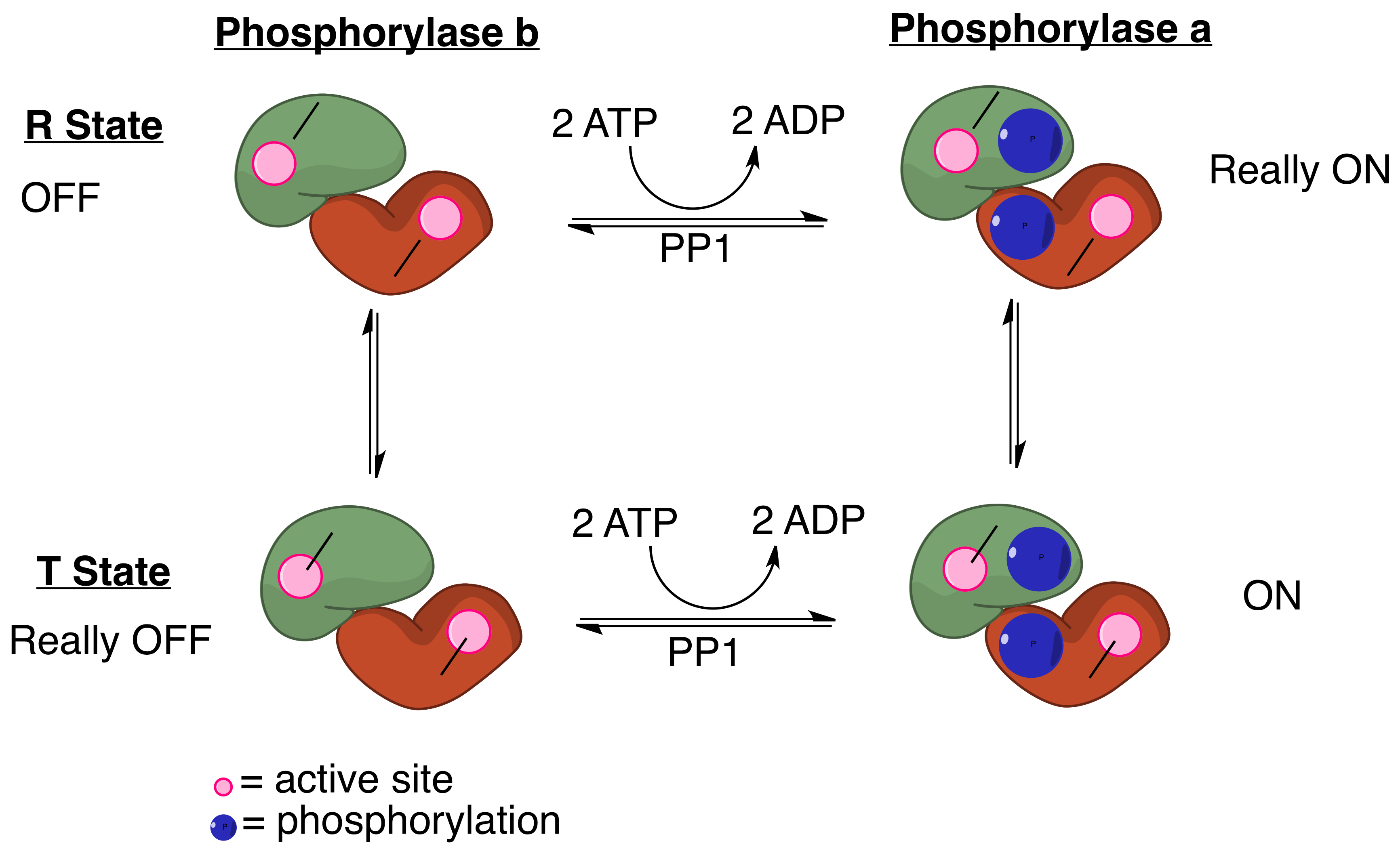
Protein Phosphatase 1
Protein phosphatase 1 (PP1) belongs to a certain class of phosphatases known as protein serine/threonine phosphatases. This type of phosphatase includes metal-dependent protein phosphatases (PPMs) and aspartate-based phosphatases. PP1 has been found to be important in the control of glycogen metabolism, muscle contraction, cell progression, neuronal activities, splicing of RNA, mitosis, cell division, apoptosis, protein synthesis, and regulation of membrane receptors and channels. Structure Each PP1 enzyme contains both a catalytic subunit and at least one regulatory subunit. The catalytic subunit consists of a 30-kD single-domain protein that can form complexes with other regulatory subunits. The catalytic subunit is highly conserved among all eukaryotes, thus suggesting a common catalytic mechanism. The catalytic subunit can form complexes with various regulatory subunits. These regulatory subunits play an important role in substrate specificity as well as compartmentalizatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
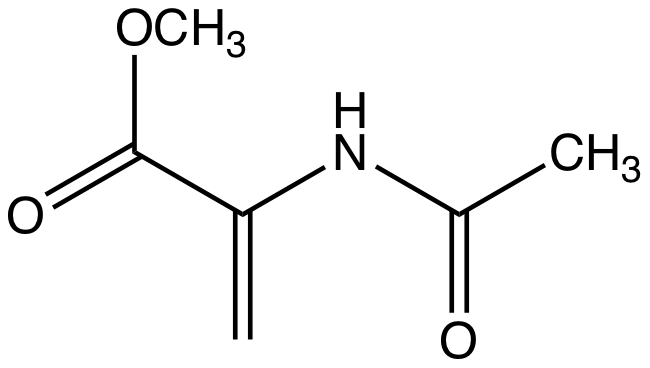
Dehydroalanine
Dehydroalanine (Cα,β-didehydroalanine, α,β-di-dehydroalanine, 2-aminoacrylate, or 2,3-didehydroalanine) is a dehydroamino acid. It does not exist in its free form, but it occurs naturally as a residue found in peptides of microbial origin. As an amino acid residue, it is unusual because it has an unsaturated backbone. Structure and reactivity Like most primary enamines, dehydroalanine is unstable. Dehydroalanine hydrolyze to pyruvate. ''N''-Acylated derivatives of dehydroalanine, such as peptides and related compounds, are stable. For example, methyl 2-acetamidoacrylate is the N-acetylated derivative of the ester. As a residue in a peptide, it is generated by a post translational modification. The required precursors are serine or cysteine residues, which undergo enzyme-mediated loss of water and hydrogen sulfide, respectively. Most amino acid residues are unreactive toward nucleophiles, but those containing dehydroalanine or some other dehydroamino acids are exceptions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydroamino Acid
In biochemistry, a dehydroamino acid is an amino acids, usually with a C=C double bond in its side chain. Dehydroamino acids are not coded by DNA, but arise via post-transcriptional modification. Examples A common dehydroamino acid is dehydroalanine, which otherwise exists only as a residue in proteins and peptides. The dehydroalanine residue is obtained dehydration of serine-containing protein/peptide (alternatively, removal of H2S from cysteine). Another example is dehydrobutyrine, derived from dehydration of threonine. Generally, amino acid residues are unreactive toward nucleophiles, but the dehydroamino acids are exceptions to this pattern. For example, dehydroalanine adds cysteine and lysine to form covalent crosslinks. An unusual dehydroamino acid is dehydroglycine (DHG) because it does not contain a carbon-carbon double bond. Instead it is the imine of glyoxalic acid. It arises by the radical-induced degradation of tyrosine. N-Acyl derivatives Dehydroamino a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term ''covalent bond'' dates from 1939. The prefix ''co-'' means ''jointly, associated in action, partnered to a lesser degree, '' etc.; thus a "co-valent bond", in essence, means that the atoms share " valence", such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Phosphatase
A protein phosphatase is a phosphatase enzyme that removes a phosphate group from the phosphorylated amino acid residue of its Substrate (biochemistry), substrate protein. Protein phosphorylation is one of the most common forms of reversible protein posttranslational modification (Post-translational modification, PTM), with up to 30% of all proteins being phosphorylated at any given time. Protein kinases (PKs) are the effectors of phosphorylation and catalyse the transfer of a γ-phosphate from ATP to specific amino acids on proteins. Several hundred PKs exist in mammals and are classified into distinct super-families. Proteins are phosphorylated predominantly on Ser, Thr and Tyr residues, which account for 79.3, 16.9 and 3.8% respectively of the phosphoproteome, at least in mammals. In contrast, protein phosphatases (PPs) are the primary effectors of dephosphorylation and can be grouped into three main classes based on sequence, structure and catalytic function. The largest class of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxic Algae Bloom In Lake Erie
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell (cytotoxicity) or an organ such as the liver (hepatotoxicity). By extension, the word may be metaphorically used to describe toxic effects on larger and more complex groups, such as the family unit or society at large. Sometimes the word is more or less synonymous with poisoning in everyday usage. A central concept of toxicology is that the effects of a toxicant are dose-dependent; even water can lead to water intoxication when taken in too high a dose, whereas for even a very toxic substance such as snake venom there is a dose below which there is no detectable toxic effect. Toxicity is species-specific, making cross-species analysis problematic. Newer paradigms and metrics are evolving to bypass a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PP2A
Protein phosphatase 2 (PP2), also known as PP2A, is an enzyme that in humans is encoded by the ''PPP2CA'' gene. The PP2A heterotrimeric protein phosphatase is ubiquitously expressed, accounting for a large fraction of phosphatase activity in eukaryotic cells. Its serine/threonine phosphatase activity has a broad substrate specificity and diverse cellular functions. Among the targets of PP2A are proteins of oncogenic signaling cascades, such as Raf, MEK, and AKT, where PP2A may act as a tumor suppressor. Structure and function PP2A consists of a dimeric core enzyme composed of the structural A and catalytic C subunits, and a regulatory B subunit. When the PP2A catalytic C subunit associates with the A and B subunits several species of holoenzymes are produced with distinct functions and characteristics. The A subunit, a founding member of the HEAT repeat protein family (huntington-elongation-A subunit-TOR), is the scaffold required for the formation of the heterotrimeric co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |