|
Methylmalonyl-CoA Mutase Deficiency
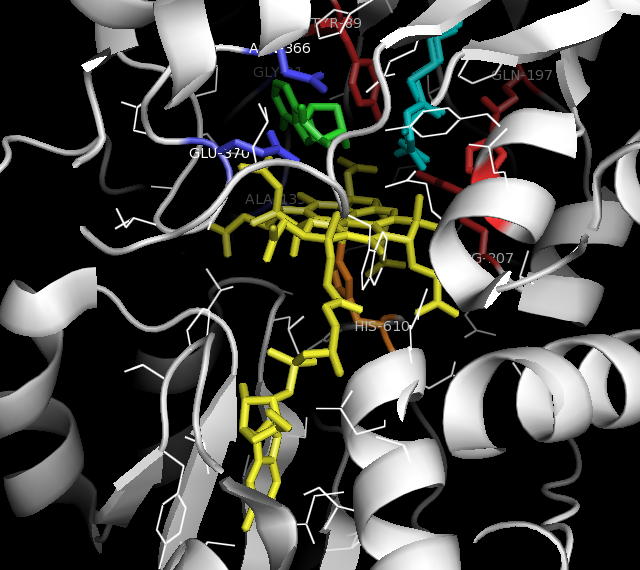
Methylmalonyl-CoA mutase is a mitochondrial homodimer apoenzyme (EC. 5. 4.99.2) that focuses on the catalysis of methylmalonyl CoA to succinyl CoA. The enzyme is bound to adenosylcobalamin, a hormonal derivative of vitamin B12 in order to function. Methylmalonyl-CoA mutase deficiency is caused by genetic defect in the MUT gene responsible for encoding the enzyme. Deficiency in this enzyme accounts for 60% of the cases of methylmalonic acidemia. Symptoms People with methylmalonyl CoA mutase deficiency exhibit many symptoms similar to other diseases involving inborn errors of metabolism. Newborn babies experience with vomiting, acidosis, hyperammonemia, hepatomegaly (enlarged livers), hyperglycinemia (high glycine levels), and hypoglycemia (low blood sugar). Later, cases of thrombocytopenia and neutropenia can occur. In some cases intellectual and developmental disabilities, such as autism, were noted with increased frequency in populations with methylmalonyl-CoA mutase deficie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylmalonyl-CoA Mutase
Methylmalonyl-CoA mutase (, MCM), mitochondrial, also known as methylmalonyl-CoA isomerase, is a protein that in humans is encoded by the ''MUT'' gene. This vitamin B12-dependent enzyme catalyzes the isomerization of methylmalonyl-CoA to succinyl-CoA in humans. Mutations in ''MUT'' gene may lead to various types of methylmalonic aciduria. MCM was first identified in rat liver and sheep kidney in 1955. In its latent form, it is 750 amino acids in length. Upon entry to the mitochondria, the 32 amino acid mitochondrial leader sequence at the N-terminus of the protein is cleaved, forming the fully processed monomer. The monomers then associate into homodimers, and bind AdoCbl (one for each monomer active site) to form the final, active holoenzyme form. Structure Gene The ''MUT'' gene lies on the chromosome location of 6p12.3 and consists of 13 exons, spanning over 35kb. Protein The mature enzyme is a homodimer with the N-terminal CoA binding domain and the C- terminal cobala ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutropenia
Neutropenia is an abnormally low concentration of neutrophils (a type of white blood cell) in the blood. Neutrophils make up the majority of circulating white blood cells and serve as the primary defense against infections by destroying bacteria, bacterial fragments and immunoglobulin-bound viruses in the blood. People with neutropenia are more susceptible to bacterial infections and, without prompt medical attention, the condition may become life-threatening ( neutropenic sepsis). Neutropenia can be divided into congenital and acquired, with severe congenital neutropenia (SCN) and cyclic neutropenia (CyN) being autosomal dominant and mostly caused by heterozygous mutations in the ELANE gene ( neutrophil elastase). Neutropenia can be acute (temporary) or chronic (long lasting). The term is sometimes used interchangeably with "leukopenia" ("deficit in the number of white blood cells"). Decreased production of neutrophils is associated with deficiencies of vitamin B12 and fol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Low-protein Diet
A low-protein diet is a diet in which people decrease their intake of protein. A low-protein diet is used as a therapy for inherited metabolic disorders, such as phenylketonuria and homocystinuria, and can also be used to treat kidney or liver disease. Low protein consumption appears to reduce the risk of bone breakage, presumably through changes in calcium homeostasis. Consequently, there is no uniform definition of what constitutes low-protein, because the amount and composition of protein for an individual with phenylketonuria would differ substantially from one with homocystinuria or tyrosinemia. History By studying the composition of food in the local population in Germany, Carl von Voit established a standard of 118 grams of protein per day. Russell Henry Chittenden showed that less than half that amount was needed to maintain good health. Protein requirement The daily requirement for humans to remain in nitrogen balance is relatively small. The median human adult req ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea Cycle 2
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid. Urea serves an important role in the metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. It is a colorless, odorless solid, highly soluble in water, and practically non-toxic ( is 15 g/kg for rats). Dissolved in water, it is neither acidic nor alkaline. The body uses it in many processes, most notably nitrogen excretion. The liver forms it by combining two ammonia molecules () with a carbon dioxide () molecule in the urea cycle. Urea is widely used in fertilizers as a source of nitrogen (N) and is an important raw material for the chemical industry. In 1828 Friedrich Wöhler Wöhler synthesis, discovered that urea can be produced from inorganic starting materials, which was an important conc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propionyl-CoA
Propionyl-CoA is a coenzyme A derivative of propionic acid. It is composed of a 24 total carbon chain (without the coenzyme, it is a 3 carbon structure) and its production and metabolic fate depend on which organism it is present in. Several different pathways can lead to its production, such as through the catabolism of specific amino acids or the oxidation of odd-chain fatty acids. It later can be broken down by propionyl-CoA carboxylase or through the methylcitrate cycle. In different organisms, however, propionyl-CoA can be sequestered into controlled regions, to alleviate its potential toxicity through accumulation. Genetic deficiencies regarding the production and breakdown of propionyl-CoA also have great clinical and human significance. Production There are several different pathways through which propionyl-CoA can be produced: * Propionyl-CoA, a three-carbon structure, is considered to be a minor species of propionic acid. Therefore, odd-number chains of fatty acids ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propanoyl-CoA
Propionyl-CoA is a coenzyme A derivative of propionic acid. It is composed of a 24 total carbon chain (without the coenzyme, it is a 3 carbon structure) and its production and metabolic fate depend on which organism it is present in. Several different pathways can lead to its production, such as through the catabolism of specific amino acids or the oxidation of odd-chain fatty acids. It later can be broken down by propionyl-CoA carboxylase or through the methylcitrate cycle. In different organisms, however, propionyl-CoA can be sequestered into controlled regions, to alleviate its potential toxicity through accumulation. Genetic deficiencies regarding the production and breakdown of propionyl-CoA also have great clinical and human significance. Production There are several different pathways through which propionyl-CoA can be produced: * Propionyl-CoA, a three-carbon structure, is considered to be a minor species of propionic acid. Therefore, odd-number chains of fatty acids are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. It is encoded by the codon AUG. Methionine is also an important part of angiogenesis, the growth of new blood vessels. Supplementation may benefit those suffering from copper poisoning. Overconsumption of methionine, the methyl group donor in DNA methylation, is related to cancer growth in a number of studies. Methionine was first isolated in 1921 by John Howard Mueller. Biochemical details Methionine (abbreviated as Met or M; encoded by the codon AUG) is an α-amino acid that is used in the biosynthesis of proteins. It contains a carboxyl group (which is in the deprotonated −COO− form under biological pH conditions), an amino group (which is in the protonat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valine
Valine (symbol Val or V) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated −NH3+ form under biological conditions), an α- carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isopropyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, beans and legumes. It is encoded by all codons starting with GU (GUU, GUC, GUA, and GUG). History and etymology Valine was first isolated from casein in 1901 by Hermann Emil Fischer. The name valine comes from valeric acid, which in turn is named after the plant valerian due to the presence of the acid in the roots of the plant. Nomenclature According to IUPAC, carbon atoms forming valine are numbered sequentia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), a carboxyl group (which is in the deprotonated −COO− form under biological conditions), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as ''E. coli''. It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG). Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices). Modifications The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can underg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoleucine
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO form under biological conditions), and a hydrocarbon side chain with a branch (a central carbon atom bound to three other carbon atoms). It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet. Isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes in other organisms such as bacteria. It is encoded by the codons AUU, AUC, and AUA. Metabolism Biosynthesis As an essential nutrient, it is not synthesized in the body, hence it must be ingested, usually as a component of proteins. In plants and microorganisms, it is synthesized via several steps, starti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |