|
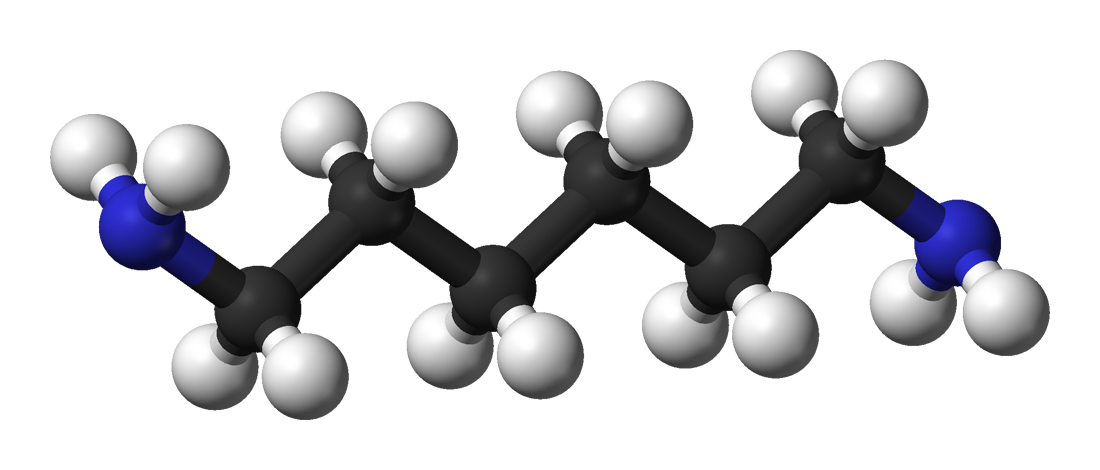
Methylidyne Group
In organic chemistry, a methylidyne group or just methylidyne is a neutral part of a molecule (a substituent or functional group) with formula , consisting of a carbon atom bonded to a hydrogen atom by one single bond and to the rest of the molecule by one triple bond. (2013''Methylidyne group''in the Chemical Entities of Biological Interest (ChEBI) database. Accessed on 2013-02-06. For example, a methylidyne group is present in ''n''-methylidyne-1-hexanaminium, . The name "methylidyne" is also used for the methylidyne radical (carbyne) , the same two atoms not bound to any other atom. See also * Methylene group or methylidene = * Methylene bridge or methanediyl −− * Methine group, methylylidene, or methanylylidene =CH− * Methanetriyl group In organic chemistry, a methine group or methine bridge is a trivalent functional group , derived formally from methane. It consists of a carbon atom bound by two single covalent bond, bonds and one double bond, where one of the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respectively). Like charges repel each other and unlike charges attract each other. An object with an absence of net charge is referred to as neutral. Early knowledge of how charged substances interact is now called classical electrodynamics, and is still accurate for problems that do not require consideration of quantum effects. Electric charge is a conserved property; the net charge of an isolated system, the amount of positive charge minus the amount of negative charge, cannot change. Electric charge is carried by subatomic particles. In ordinary matter, negative charge is carried by electrons, and positive charge is carried by the protons in the nuclei of atoms. If there are more electrons than protons in a piece of matter, it will have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not consid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side chain'' and '' pendant group'', are used almost interchangeably to describe those branches from the parent structure, though certain distinctions are made in polymer chemistry. In polymers, side chains extend from the backbone structure. In proteins, side chains are attached to the alpha carbon atoms of the amino acid backbone.) The suffix ''-yl'' is used when naming organic compounds that contain a single bond replacing one hydrogen; ''-ylidene'' and ''-ylidyne'' are used with double bonds and triple bonds, respectively. In addition, when naming hydrocarbons that contain a substituent, positional numbers are used to indicate which carbon atom the substituent attaches to when such information is needed to distinguish between isomers. Su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an ...—its atom making four electrons available to form covalent bond, covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, Carbon-12, C and Carbon-13, C being stable, while Carbon-14, C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the Timeline of chemical element discoveries#Ancient discoveries, few elements known since antiquity. Carbon is the 15th Abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the Abundance of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an atom are a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylidyne Radical
Methylidyne, or (unsubstituted) carbyne, is an organic compound whose molecule consists of a single hydrogen atom bonded to a carbon atom. It is the parent compound of the carbynes, which can be seen as obtained from it by substitution of other functional groups for the hydrogen. The carbon atom is left with either one or three unpaired electrons (unsatisfied valence bonds), depending on the molecule's excitation state; making it a radical. Accordingly, the chemical formula can be CH• or CH3• (also written as ⫶CH); each dot representing an unpaired electron. The corresponding systematic names are methylylidene or hydridocarbon(•), and methanetriyl or hydridocarbon(3•). However, the formula is often written simply as CH. Methylidyne is a highly reactive gas, that is quickly destroyed in ordinary conditions but is abundant in the interstellar medium (and was one of the first molecules to be detected there). Nomenclature The trivial name ''carbyne'' is the preferre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylene Group
In organic chemistry, a methylene group is any part of a molecule that consists of two hydrogen atoms bound to a carbon atom, which is connected to the remainder of the molecule by two single bonds. The group may be represented as , where the '<' denotes the two bonds. This can equally well be represented as . This stands in contrast to a situation where the carbon atom is bound to the rest of the molecule by a double bond, which is preferably called a , represented . Formerly the methylene name was used for both isomers. The name ““ can be used for the single-bonded isomer, to emphatically exclude methylidene. The distinction is often important, because the double bond is chemically di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylene Bridge
In organic chemistry, a methylene bridge, methylene spacer, or methanediyl group is any part of a molecule with formula ; namely, a carbon atom bound to two hydrogen atoms and connected by single bonds to two other distinct atoms in the rest of the molecule. It is the repeating unit in the skeleton of the unbranched alkanes. A methylene bridge can also act as a bidentate ligand joining two metals in a coordination compound, such as titanium and aluminum in Tebbe's reagent.W. A. Herrmann (1982), "The methylene bridge". In ''Advances in Organometallic Chemistry'', volume 20, pages 195-197. A methylene bridge is often called a methylene group or simply methylene, as in "methylene chloride" (dichloromethane ). As a bridge in other compounds, for example in cyclic compounds, it is given the name methano. However, the term methylene group (or "methylidene") properly applies to the group when it is connected to the rest of the molecule by a double bond (), giving it chemical proper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



9.png)