|
Methyl-parathion
Parathion, also called parathion-ethyl or diethyl parathion and locally known as "Folidol", is an organophosphate insecticide and acaricide. It was originally developed by IG Farben in the 1940s. It is highly toxic to non-target organisms, including humans, so its use has been banned or restricted in most countries. The basic structure is shared by parathion methyl. History Parathion was developed by Gerhard Schrader for the German trust IG Farben in the 1940s. After World War II and the collapse of IG Farben due to the war crime trials, the Western allies seized the patent, and parathion was marketed worldwide by different companies and under different brand names. The most common German brand was E605 (banned in Germany after 2002); this was not a food-additive "E number" as used in the EU today. "E" stands for ''Entwicklungsnummer'' (German for "development number"). It is an irreversible acetylcholinesterase inhibitor. Safety concerns have later led to the development of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parathion Methyl
Parathion methyl, or methyl parathion, is an organophosphate insecticide, possessing an organothiophosphate group. It is structurally very similar to parathion-ethyl. It is not allowed for sale and import in nearly all countries around the world, while a few allow it under subject to specified conditions only. Applications Parathion methyl is used as an insecticide on crops, including cotton. Trade Names Penncap-M, Metacide Safety People can be exposed to parathion methyl in the workplace by breathing it in, getting it on their skin, swallowing it, or getting it in their eyes. Since it is an acetylcholinesterase inhibitor, symptoms of exposure to parathion methyl include irritated eyes and skin, nausea and vomiting, abdominal pain, diarrhea, salivation, feeling weak and tired, headache, runny nose, tightness in the chest, blurry vision, pupil constriction, irregular heartbeat, muscle twitches (fasciculation), and difficulty breathing Shortness of breath (SOB), a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xylene
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are substituted determines which of three structural isomers results. It is a colorless, flammable, slightly greasy liquid of great industrial value. The mixture is referred to as both xylene and, more precisely, xylenes. Mixed xylenes refers to a mixture of the xylenes plus ethylbenzene. The four compounds have identical empirical formulas . Typically the four compounds are produced together by various catalytic reforming and pyrolysis methods. Occurrence and production Xylenes are an important petrochemical produced by catalytic reforming and also by coal carbonisation in the manufacture of coke fuel. They also occur in crude oil in concentrations of about 0.5–1%, depending on the source. Small quantities occur in gasoline and aircraft f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Dithiophosphoric Acid
Diethyl dithiophosphoric acid, sometimes mistakenly called diethyl dithiophosphate, is the organophosphorus compound with the formula (C2H5O)2PS2H. It is the processor for production of the organophosphate insecticide Terbufos. Although samples can appear dark, it is a colorless liquid.J. Svara, N. Weferling, T. Hofmann "Phosphorus Compounds, Organic" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2006. It is prepared by treating phosphorus pentasulfide with ethanol: :P2S5 + 4 C2H5OH → 2 (C2H5O)2PS2H + H2S Reactions : Diethyl- and dimethyl dithiophosphoric acids react with bases. The results of this neutralization reaction are salts, e.g., ammonium diethyl dithiophosphate. Diethyl dithiophosphoric acid reacts with zinc oxide to give zinc dithiophosphate Zinc dialkyldithiophosphates (often referred to as ZDDP) are a family of coordination compounds developed in the 1940s that feature zinc bound to the anion of a dialkyldithiophos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and water molecule to split into two parts. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylcholinesterase Inhibitor Resistance
Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and duration of action of acetylcholine in the central nervous system, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being butyryl-cholinesterase inhibitors. Acetylcholinesterase is the primary member of the cholinesterase enzyme family. Acetylcholinesterase inhibitors are classified as reversible, irreversible, or quasi-irreversible (also called pseudo-irreversible). Mechanism of action Organophosphates Organophosphates like TEPP and sarin inhibit cholinesterases, enzymes that hydrolyze the neurotransmitter acetylcholine. The active centre of cholinesterases feature two important sites, namely ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paraoxon
Paraoxon is a parasympathomimetic which acts as an cholinesterase inhibitor. It is an organophosphate oxon, and the active metabolite of the insecticide parathion. It is also used as an ophthalmological drug against glaucoma. Paraoxon is one of the most potent acetylcholinesterase-inhibiting insecticides available, around 70% as potent as the nerve agent sarin, and so is now rarely used as an insecticide due to the risk of poisoning to humans and other animals. Paraoxon has been used by scientists to study acute and chronic effects of organophosphate intoxication. It is easily absorbed through skin, and was allegedly used as an assassination weapon by the apartheid-era South Africa South Africa, officially the Republic of South Africa (RSA), is the southernmost country in Africa. It is bounded to the south by of coastline that stretch along the South Atlantic and Indian Oceans; to the north by the neighbouring count ...n chemical weapons program Project Coast. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidase
In biochemistry, an oxidase is an enzyme that catalyzes oxidation-reduction reactions, especially one involving dioxygen (O2) as the electron acceptor. In reactions involving donation of a hydrogen atom, oxygen is reduced to water (H2O) or hydrogen peroxide (H2O2). Some oxidation reactions, such as those involving monoamine oxidase or xanthine oxidase, typically do not involve free molecular oxygen. The oxidases are a subclass of the oxidoreductases. Examples An important example is cytochrome c oxidase, the key enzyme that allows the body to employ oxygen in the generation of energy and the final component of the electron transfer chain. Other examples are: * Glucose oxidase * Monoamine oxidase * Cytochrome P450 oxidase * NADPH oxidase * Xanthine oxidase * L-gulonolactone oxidase * Laccase * Lysyl oxidase * Polyphenol oxidase * Sulfhydryl oxidase. This enzyme oxidises thiol groups. Oxidase test In microbiology, the oxidase test is used as a phenotypic characteristic for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
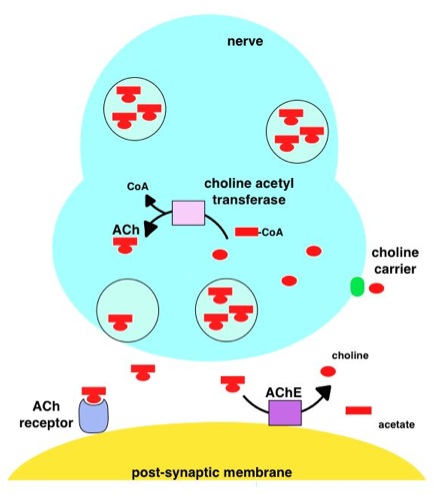
Acetylcholinesterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters: : acetylcholine + H2O = choline + acetate It is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate synaptic transmission. It belongs to the carboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides. Enzyme structure and mechanism AChE is a hydrolase that hydrolyzes choline esters. It has a very high catalytic activity—each molecule of AChE degrades about 25,000 molecules of acetylcholine (ACh) per second, approaching the limit allowed by diffusion of the substrate. The active site of AChE comprises 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fruit
In botany, a fruit is the seed-bearing structure in flowering plants that is formed from the ovary after flowering. Fruits are the means by which flowering plants (also known as angiosperms) disseminate their seeds. Edible fruits in particular have long propagated using the movements of humans and animals in a symbiotic relationship that is the means for seed dispersal for the one group and nutrition for the other; in fact, humans and many animals have become dependent on fruits as a source of food. Consequently, fruits account for a substantial fraction of the world's agricultural output, and some (such as the apple and the pomegranate) have acquired extensive cultural and symbolic meanings. In common language usage, "fruit" normally means the seed-associated fleshy structures (or produce) of plants that typically are sweet or sour and edible in the raw state, such as apples, bananas, grapes, lemons, oranges, and strawberries. In botanical usage, the term "fruit" als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rice
Rice is the seed of the grass species '' Oryza sativa'' (Asian rice) or less commonly '' Oryza glaberrima'' (African rice). The name wild rice is usually used for species of the genera '' Zizania'' and ''Porteresia'', both wild and domesticated, although the term may also be used for primitive or uncultivated varieties of '' Oryza''. As a cereal grain, domesticated rice is the most widely consumed staple food for over half of the world's human population,Abstract, "Rice feeds more than half the world's population." especially in Asia and Africa. It is the agricultural commodity with the third-highest worldwide production, after sugarcane and maize. Since sizable portions of sugarcane and maize crops are used for purposes other than human consumption, rice is the most important food crop with regard to human nutrition and caloric intake, providing more than one-fifth of the calories consumed worldwide by humans. There are many varieties of rice and culinary preferences t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cotton
Cotton is a soft, fluffy staple fiber that grows in a boll, or protective case, around the seeds of the cotton plants of the genus '' Gossypium'' in the mallow family Malvaceae. The fiber is almost pure cellulose, and can contain minor percentages of waxes, fats, pectins, and water. Under natural conditions, the cotton bolls will increase the dispersal of the seeds. The plant is a shrub native to tropical and subtropical regions around the world, including the Americas, Africa, Egypt and India. The greatest diversity of wild cotton species is found in Mexico, followed by Australia and Africa. Cotton was independently domesticated in the Old and New Worlds. The fiber is most often spun into yarn or thread and used to make a soft, breathable, and durable textile. The use of cotton for fabric is known to date to prehistoric times; fragments of cotton fabric dated to the fifth millennium BC have been found in the Indus Valley civilization, as well as fabric remnants date ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

2.png)