|
Medroxalol
Medroxalol is a vasodilator beta blocker also classified as a mixed receptor blocker as it blocks both alpha and beta receptors. Synthesis For the first step, salicylamide (1) is the subject of a Friedel-Crafts acetylation and then the aromatic methylketone is halogenated. in the usual manner. The bromide in 2 is then displaced by the nitrogen in ''N''-benzyl-1-(3',4'-methylenedioxyphenyl)-3-butylamine (3), which is itself prepared by reductive amination on the corresponding ketone. The product of the last step (4) is catalytically hydrogenated. This serves the dual purpose both of reducing the ketone and removing the benzyl protecting group affording the product medroxalol (5). Note that a benzyl protecting group is not necessarily used. See also * Labetalol Labetalol is a medication used to treat high blood pressure and in long term management of angina. This includes essential hypertension, hypertensive emergencies, and hypertension of pregnancy. In essential hypert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medroxalol Synthesis
Medroxalol is a vasodilator beta blocker also classified as a mixed receptor blocker as it blocks both alpha and beta receptors. Synthesis For the first step, salicylamide Salicylamide (''o''-hydroxybenzamide or amide of salicyl) is a non-prescription drug with analgesic and antipyretic properties. Its medicinal uses are similar to those of aspirin. Salicylamide is used in combination with both aspirin and caffe ... (1) is the subject of a Friedel-Crafts acetylation and then the aromatic methylketone is halogenated. in the usual manner. The bromide in 2 is then displaced by the nitrogen in ''N''-benzyl-1-(3',4'-methylenedioxyphenyl)-3-butylamine (3), which is itself prepared by reductive amination on the corresponding ketone. The product of the last step (4) is catalytically hydrogenated. This serves the dual purpose both of reducing the ketone and removing the benzyl protecting group affording the product medroxalol (5). Note that a benzyl protecting group is not nec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vasodilator
Vasodilation is the widening of blood vessels. It results from relaxation of smooth muscle cells within the vessel walls, in particular in the large veins, large arteries, and smaller arterioles. The process is the opposite of vasoconstriction, which is the narrowing of blood vessels. When blood vessels dilate, the flow of blood is increased due to a decrease in vascular resistance and increase in cardiac output. Therefore, dilation of arterial blood vessels (mainly the arterioles) decreases blood pressure. The response may be intrinsic (due to local processes in the surrounding tissue) or extrinsic (due to hormones or the nervous system). In addition, the response may be localized to a specific organ (depending on the metabolic needs of a particular tissue, as during strenuous exercise), or it may be systemic (seen throughout the entire systemic circulation). Endogenous substances and drugs that cause vasodilation are termed vasodilators. Such vasoactivity is necessary for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Blocker
Beta blockers, also spelled β-blockers, are a class of medications that are predominantly used to manage cardiac arrhythmia, abnormal heart rhythms, and to protect the heart from a second myocardial infarction, heart attack after a first heart attack (preventative healthcare, secondary prevention). They are also widely used to treat hypertension, high blood pressure, although they are no longer the first choice for initial treatment of most patients. Beta blockers are competitive antagonists that block the receptor sites for the endogenous catecholamines Adrenaline, epinephrine (adrenaline) and norepinephrine (noradrenaline) on beta receptor, adrenergic beta receptors, of the sympathetic nervous system, which mediates the fight-or-flight response. Some block activation of all types of β-adrenergic receptors and others are selective for one of the three known types of beta receptors, designated β1, β2 and β3 receptors. Beta-1 adrenergic receptor, β1-adrenergic receptors are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salicylamide
Salicylamide (''o''-hydroxybenzamide or amide of salicyl) is a non-prescription drug with analgesic and antipyretic properties. Its medicinal uses are similar to those of aspirin. Salicylamide is used in combination with both aspirin and caffeine in the over-the-counter pain remedy PainAid. It was also an ingredient in the over-the-counter pain remedy BC Powder but was removed from the formulation in 2009, and Excedrin used the ingredient from 1960 to 1980 in conjunction with aspirin, acetaminophen, and caffeine. It was used in later formulations of Vincent's powders in Australia as a substitute for phenacetin. Derivatives Derivatives of salicylamide include ethenzamide, labetalol, medroxalol, lopirin, otilonium, oxyclozanide, salicylanilide, niclosamide, and raclopride. See also * 4-Aminosalicylic acid *Mesalazine *Salsalate Salsalate is a medication that belongs to the salicylate and nonsteroidal anti-inflammatory drug (NSAID) classes. Salsalate is the gener ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylketone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour. Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in |
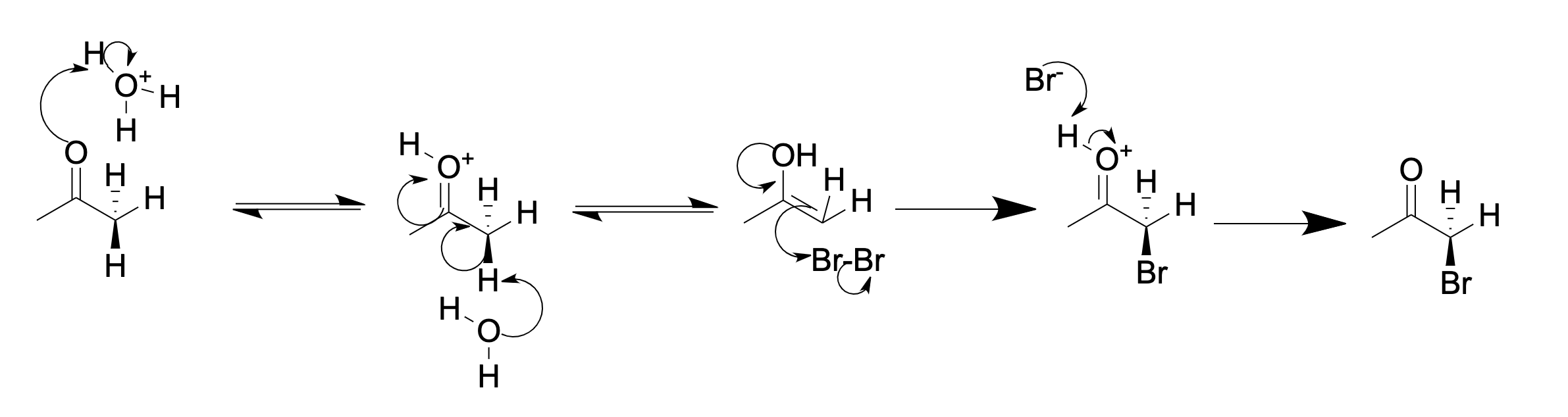
Ketone Halogenation
In organic chemistry, α-keto halogenation is a special type of halogenation. The reaction may be carried out under either acidic or basic conditions in an aqueous medium with the corresponding elemental halogen. In this way, chloride, bromide, and iodide (but notably not fluoride) functionality can be inserted selectively in the alpha position of a ketone. The position alpha to the carbonyl group in a ketone is easily halogenated. This is due to its ability to form an enolate in basic solution, or an enol in acidic solution. An example of alpha halogenation is the mono-bromination of acetone, carried out under either acidic or basic conditions, to give bromoacetone: Acidic (in acetic acid): Basic (in aqueous NaOH): In acidic solution, usually only one alpha hydrogen is replaced by a halogen, as each successive halogenation is slower than the first. The halogen decreases the basicity of the carbonyl oxygen, thus making protonation less favorable. However, in basic solut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reductive Amination
Reductive amination (also known as reductive alkylation) is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is considered the most important way to make amines, and a majority of amines made in the pharmaceutical industry are made this way. Reaction process In this organic reaction, the amine first reacts with the carbonyl group to form a hemiaminal species, which subsequently loses one molecule of water in a reversible manner by alkylimino-de-oxo-bisubstitution, to form the imine. The equilibrium between aldehyde/ketone and imine can be shifted toward imine formation by removal of the formed water through physical or chemical means. This intermediate imine can then be isolated and reduced with a suitable reducing agent (e.g., sodium borohydride). This method is sometimes called indirect reductive amination. In a separate approach, imine formation and redu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons. Process Hydrogenation has three components, the unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same catalysts and conditions that are used for hydrogenation reactions can also lead to isomerization of the alkenes from cis to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Labetalol
Labetalol is a medication used to treat high blood pressure and in long term management of angina. This includes essential hypertension, hypertensive emergencies, and hypertension of pregnancy. In essential hypertension it is generally less preferred than a number of other blood pressure medications. It can be given by mouth or by injection into a vein. Common side effects include low blood pressure with standing, dizziness, feeling tired, and nausea. Serious side effects may include low blood pressure, liver problems, heart failure, and bronchospasm. Use appears safe in the latter part of pregnancy and it is not expected to cause problems during breastfeeding. It works by blocking the activation of β-receptors and α-receptors. Labetalol was patented in 1966 and came into medical use in 1977. It is available as a generic medication. In 2020, it was the 210th most commonly prescribed medication in the United States, with more than 2million prescriptions. Medical uses Lab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |