|
Medical Food
Medical foods are foods that are specially formulated and intended for the dietary management of a disease that has distinctive nutritional needs that cannot be met by normal diet alone. In the United States they were defined in the Food and Drug Administration's 1988 Orphan Drug Act Amendments and are subject to the general food and safety labeling requirements of the Federal Food, Drug, and Cosmetic Act. In Europe the European Food Safety Authority established definitions for "foods for special medical purposes" (FSMPs) in 2015. Definition Medical foods, called "food for special medical purposes" in Europe, are distinct from the broader category of foods for special dietary use, from traditional foods that bear a health claim, and from dietary supplements. In order to be considered a medical food the product must, at a minimum: * be a food for oral ingestion or tube feeding (nasogastric tube) * be labeled for the dietary management of a specific medical disorder, disease or condit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Novartis Fibersource HN Medical Food On IV Pole
Novartis AG is a Swiss-American multinational pharmaceutical corporation based in Basel, Switzerland and Cambridge, Massachusetts, United States (global research).name="novartis.com">https://www.novartis.com/research-development/research-locations It is one of the largest pharmaceutical companies in the world. Novartis manufactures the drugs clozapine (Clozaril), diclofenac (Voltaren; sold to GlaxoSmithKline in 2015 deal), carbamazepine (Tegretol), valsartan (Diovan), imatinib mesylate (Gleevec/Glivec), cyclosporine (Neoral/Sandimmune), letrozole (Femara), methylphenidate (Ritalin; production ceased 2020), terbinafine (Lamisil), deferasirox (Exjade), and others. In March 1996, the companies Ciba-Geigy and Sandoz merged to form Novartis; the pharmaceutical and agrochemical divisions of both companies formed Novartis as an independent entity. Other Ciba-Geigy and Sandoz businesses were sold, or, like Ciba Specialty Chemicals, spun off as independent companies. The Sandoz brand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Federal Food, Drug, And Cosmetic Act
The United States Federal Food, Drug, and Cosmetic Act (abbreviated as FFDCA, FDCA, or FD&C) is a set of laws passed by the United States Congress in 1938 giving authority to the U.S. Food and Drug Administration (FDA) to oversee the safety of food, drugs, medical devices, and cosmetics. A principal author of this law was Royal S. Copeland, a three-term U.S. senator from New York. In 1968, the Electronic Product Radiation Control provisions were added to the FD&C. Also in that year the FDA formed the Drug Efficacy Study Implementation (DESI) to incorporate into FD&C regulations the recommendations from a National Academy of Sciences investigation of effectiveness of previously marketed drugs. The act has been amended many times, most recently to add requirements about bioterrorism preparations. The introduction of this act was influenced by the death of more than 100 patients due to elixir sulfanilamide, a sulfanilamide medication where the toxic solvent diethylene glycol was us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Food Safety Authority
The European Food Safety Authority (EFSA) is the agency of the European Union (EU) that provides independent scientific advice and communicates on existing and emerging risks associated with the food chain. EFSA was established in February 2002, is based in Parma, Italy, and for 2021 it has a budget of €118.6 million, and a total staff of 542. The work of EFSA covers all matters with a direct or indirect impact on food and feed safety, including animal health and welfare, plant protection and plant health and nutrition. EFSA supports the European Commission, the European Parliament and EU member states in taking effective and timely risk management decisions that ensure the protection of the health of European consumers and the safety of the food and feed chain. EFSA also communicates to the public in an open and transparent way on all matters within its remit. Structure Based on a regulation of 2002, the EFSA is composed of four bodies: * Management Board * Executive Dir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Health Claim
A health claim on a food label and in food marketing is a claim by a manufacturer of food products that their food will reduce the risk of developing a disease or condition. For example, it is claimed by the manufacturers of oat cereals that oat bran can reduce cholesterol, which will lower the chances of developing serious heart conditions. Vague health claims include that the food inside is "healthy," "organic," "low fat," "non-GMO," "no sugar added," or "natural". Health claims are also made for over-the-counter drugs and prescription drugs, medical procedures, and medical devices, but these generally have a separate, much more stringent set of regulations. Health claims in the United States In the United States, health claims on Nutrition facts label#United States, nutrition facts labels are regulated by the U.S. Food and Drug Administration (FDA), while advertising is regulated by the Federal Trade Commission. Dietary supplements are regulated as a separate type of co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nasogastric Tube
Nasogastric intubation is a medical process involving the insertion of a plastic tube (nasogastric tube or NG tube) through the nose, down the oesophagus, and down into the stomach. Orogastric intubation is a similar process involving the insertion of a plastic tube (orogastric tube) through the mouth. Abraham Louis Levin invented the NG tube. Nasogastric tube is also known as Ryle's tube in Commonwealth countries, after John Alfred Ryle. Uses A nasogastric tube is used for feeding and administering drugs and other oral agents such as activated charcoal. For drugs and for minimal quantities of liquid, a syringe is used for injection into the tube. For continuous feeding, a gravity based system is employed, with the solution placed higher than the patient's stomach. If accrued supervision is required for the feeding, the tube is often connected to an electronic pump which can control and measure the patient's intake and signal any interruption in the feeding. Nasogastric tubes may ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolic Disorders
A metabolic disorder is a disorder that negatively alters the body's processing and distribution of macronutrients, such as Protein, proteins, Fat, fats, and Carbohydrate, carbohydrates. Metabolic disorders can happen when abnormal chemical reactions in the body alter the normal metabolic process. It can also be defined as inherited single gene anomaly, most of which are Dominance (genetics), autosomal recessive. Signs and symptoms Some of the symptoms that can occur with metabolic disorders are lethargy, weight loss, jaundice and seizures. The symptoms expressed would vary with the type of metabolic disorder. There are four categories of symptoms: acute symptoms, late-onset acute symptoms, progressive general symptoms and permanent symptoms. Causes Inherited metabolic disorders are one cause of metabolic disorders, and occur when a defective gene causes an enzyme deficiency. These diseases, of which there are many subtypes, are known as inborn errors of metabolism. Metabolic d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
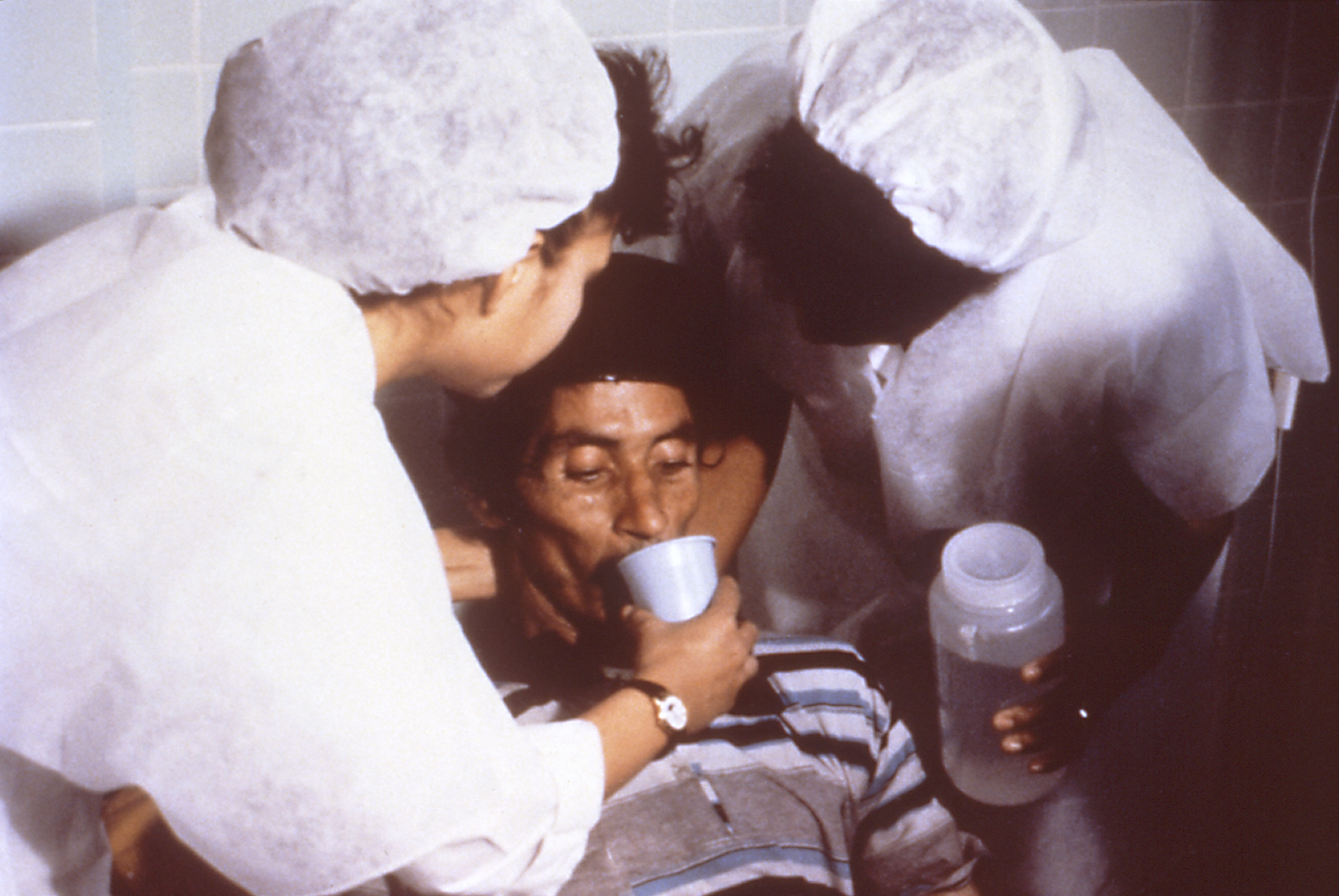
Oral Rehydration Therapy
Oral rehydration therapy (ORT) is a type of fluid replacement used to prevent and treat dehydration, especially due to diarrhea. It involves drinking water with modest amounts of sugar and salts, specifically sodium and potassium. Oral rehydration therapy can also be given by a nasogastric tube. Therapy should routinely include the use of zinc supplements. Use of oral rehydration therapy has been estimated to decrease the risk of death from diarrhea by up to 93%. Side effects may include vomiting, high blood sodium, or high blood potassium. If vomiting occurs, it is recommended that use be paused for 10 minutes and then gradually restarted. The recommended formulation includes sodium chloride, sodium citrate, potassium chloride, and glucose. Glucose may be replaced by sucrose and sodium citrate may be replaced by sodium bicarbonate, if not available. It works as glucose increases the uptake of sodium and thus water by the intestines. A number of other formulations are also ava ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
US Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed and veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not directly related to food or drugs, but involves such things as regulating lasers, cellular phones, and condoms, as well as control of disease in contexts varying from h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug Act Of 1983
The Orphan Drug Act of 1983 is a law passed in the United States to facilitate development of orphan drugs—drugs for rare diseases such as Huntington's disease, myoclonus, ALS, Tourette syndrome and muscular dystrophy which affect small numbers of individuals residing in the United States. Orphan drug designation does not indicate that the therapeutic is either safe and effective or legal to manufacture and market in the United States. That process is handled through other offices in the US Food and Drug Administration. Instead, the designation means only that the sponsor qualifies for certain benefits from the federal government, such as market exclusivity and reduced taxes. In 1982 an informal coalition of supporters and families of patients with rare diseases who formed National Organization for Rare Disorders (NORD) and others, called for change to legislation to support development of orphan drugs, or drugs for treating rare diseases. They succeeded in getting the United St ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Nutrition
Clinical nutrition centers on the prevention, diagnosis, and management of nutritional changes in patients linked to chronic diseases and conditions primarily in health care. Clinical in this sense refers to the management of patients, including not only outpatients at clinics and in private practice, but also inpatients in hospitals. It incorporates primarily the scientific fields of nutrition and dietetics. Furthermore, clinical nutrition aims to maintain a healthy energy balance, while also providing sufficient amounts of nutrients such as protein, vitamins, and minerals to patients. Dietary needs and disease processes Normally, individuals obtain the necessary nutrients their bodies require through normal daily diets that process the foods accordingly within the body. Nevertheless, there are circumstances such as disease, distress, stress, and so on that may prevent the body from obtaining sufficient nutrients through diets alone. In such conditions, a dietary supplementation sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parenteral Nutrition
Parenteral nutrition (PN) is the feeding of nutritional products to a person intravenously, bypassing the usual process of eating and digestion. The products are made by pharmaceutical compounding companies. The person receives a nutritional mix according to a formula including glucose, salts, amino acids, lipids and vitamins and dietary minerals. It is called total parenteral nutrition (TPN) or total nutrient admixture (TNA) when no significant nutrition is obtained by other routes, and partial parenteral nutrition (PPN) when nutrition is also partially enteric. It is called peripheral parenteral nutrition (PPN) when administered through vein access in a limb rather than through a central vein as central venous nutrition (CVN). Medical uses Total parenteral nutrition (TPN) is provided when the gastrointestinal tract is nonfunctional because of an interruption in its continuity (it is blocked, or has a leak – a fistula) or because its absorptive capacity is impaired.Kozier, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)