|
Magnetochemistry
Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electrons are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin-orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinides, spin-orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins. Magnetic susceptibil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, diamagnetic materials are repelled by magnetic fields and form induced magnetic fields in the direction opposite to that of the applied magnetic field. Paramagnetic materials include most chemical elements and some compounds; they have a relative magnetic permeability slightly greater than 1 (i.e., a small positive magnetic susceptibility) and hence are attracted to magnetic fields. The magnetic moment induced by the applied field is linear in the field strength and rather weak. It typically requires a sensitive analytical balance to detect the effect and modern measurements on paramagnetic materials are often conducted with a SQUID magnetometer. Paramagnetism is due to the presence of unpaired electrons in the material, so most atoms wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can use d orbitals as valence orbitals to form chemical bonds. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. Since they are metals, they are lustrous and have good electrical and thermal conductivity. Most (with the exception of group 11 and group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such as coordination complexes and oxides. Most are strongly param ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted by a magnetic field. Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In paramagnetic and ferromagnetic substances, the weak diamagnetic force is overcome by the attractive force of magnetic dipoles in the material. The magnetic permeability of diamagnetic materials is less than the permeability of vacuum, ''μ''0. In most materials, diamagnetism is a weak effect which can be detected only by sensitive laboratory instruments, but a superconductor acts as a strong diamagnet because it repels a magnetic field entirely from its interior. Diamagnetism was first discovered when Anton Brugmans observed in 1778 that bismuth was repel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
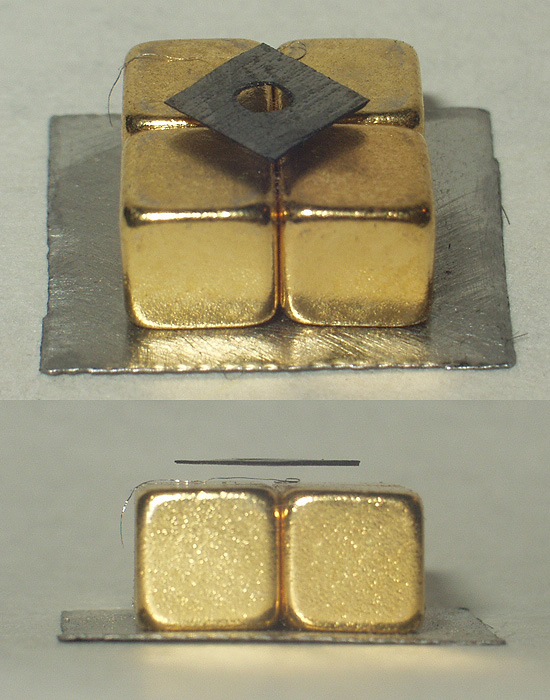
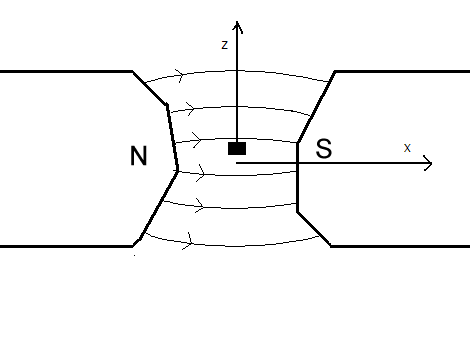
Faraday Balance
A Faraday balance is a device for measuring magnetic susceptibility. Magnetic susceptibility is related to the force experienced by a substance in a magnetic field. Various practical devices are available for the measurement of susceptibility, which differ in the shape of the magnetic field and the way the force is measured. In the Faraday balance the field is homogeneous. The pole pieces of the magnet are so shaped that there is a region in which the product of the field strength and field gradient in the ''z'' direction is constant. The sample is placed in this region. The force in this case is independent of the packing of the sample and depends only on the total mass of the material present. The method is sensitive and highly reproducible and can be applied to single crystals. The force is measured as a weight change, using a torsion balance. An alternative method for measuring magnetic susceptibility is the Gouy balance The Gouy balance, invented by the French physicist Lo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gouy Balance
The Gouy balance, invented by the French physicist Louis Georges Gouy, is a device for measuring the magnetic susceptibility of a sample. Background Amongst a wide range of interest in optics, Brownian motion, and experimental physics, Gouy also had a strong intrigue for the phenomena of magnetism. Gouy derived a mathematical expression showing that force is proportional to volume susceptibility for the interaction of material in a uniform magnetic field in 1889. From this derivation, Gouy proposed that balance measurements taken for tubes of material suspended in a magnetic field could evaluate his expression for volume susceptibility. Though Gouy never tested the scientific suggestion himself, this simple and inexpensive method became the foundation for measuring magnetic susceptibility and the blueprint for the Gouy balance. Procedure The Gouy balance measures the apparent change in the mass of the sample as it is repelled or attracted by the region of high magnetic field bet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical Balance
An analytical balance (or chemical ''balance'') is a class of balance designed to measure small mass in the sub-milligram range. The measuring pan of an analytical balance (0.1 mg resolution or better) is inside a transparent enclosure with doors so that dust does not collect and so any air currents in the room do not affect the balance's operation. This enclosure is often called a draft shield. The use of a mechanically vented balance safety enclosure, which has uniquely designed acrylic airfoils, allows a smooth turbulence-free airflow that prevents balance fluctuation and the measure of mass down to 1 μg without fluctuations or loss of product. Also, the sample must be at room temperature to prevent natural convection from forming air currents inside the enclosure from causing an error in reading. Single pan mechanical substitution balance is a method of maintaining consistent response throughout the useful capacity of the balance. This is achieved by maintaining a constant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematically, density is defined as mass divided by volume: : \rho = \frac where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate – this quantity is more specifically called specific weight. For a pure substance the density has the same numerical value as its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium and iridium are the densest known elements at standard conditions for temperature and pressure. To simplify comparisons of density across different s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molar Mass
In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance which is the number of moles in that sample, measured in moles. The molar mass is a bulk, not molecular, property of a substance. The molar mass is an ''average'' of many instances of the compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk quantities. The molecular mass and formula mass are commonly used as a synonym of molar mass, particularly for molecular compounds; however, the most authoritative sources define it differently. The difference is that molecular mass is the mass of one specific particle or molecul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gouy Bal
{{disambig, geo, surname ...
__NOTOC__ Gouy may refer to: Places Belgium * Gouy-lez-Piéton Communes in France * Gouy, Aisne, in the department of Aisne * Gouy, Seine-Maritime, in the department of Seine-Maritime * Gouy-en-Artois, in the department of Pas-de-Calais * Gouy-en-Ternois, in the department of Pas-de-Calais * Gouy-les-Groseillers, in the department of Oise * Gouy-Saint-André, in the department of Pas-de-Calais * Gouy-Servins, in the department of Pas-de-Calais * Gouy-sous-Bellonne, in the department of Pas-de-Calais Other uses *Louis Georges Gouy, a French physicist **Gouy balance The Gouy balance, invented by the French physicist Louis Georges Gouy, is a device for measuring the magnetic susceptibility of a sample. Background Amongst a wide range of interest in optics, Brownian motion, and experimental physics, Gouy als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calibrated
In measurement technology and metrology, calibration is the comparison of measurement values delivered by a device under test with those of a calibration standard of known accuracy. Such a standard could be another measurement device of known accuracy, a device generating the quantity to be measured such as a voltage, a sound tone, or a physical artifact, such as a meter ruler. The outcome of the comparison can result in one of the following: * no significant error being noted on the device under test * a significant error being noted but no adjustment made * an adjustment made to correct the error to an acceptable level Strictly speaking, the term "calibration" means just the act of comparison and does not include any subsequent adjustment. The calibration standard is normally traceable to a national or international standard held by a metrology body. BIPM Definition The formal definition of calibration by the International Bureau of Weights and Measures (BIPM) is the follo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cryostat
A cryostat (from ''cryo'' meaning cold and ''stat'' meaning stable) is a device used to maintain low cryogenic temperatures of samples or devices mounted within the cryostat. Low temperatures may be maintained within a cryostat by using various refrigeration methods, most commonly using cryogenic fluid bath such as liquid helium. Hence it is usually assembled into a vessel, similar in construction to a vacuum flask or Dewar. Cryostats have numerous applications within science, engineering, and medicine. Types Closed-cycle cryostats Closed-cycle cryostats consist of a chamber through which cold helium vapour is pumped. An external mechanical refrigerator extracts the warmer helium exhaust vapour, which is cooled and recycled. Closed-cycle cryostats consume a relatively large amount of electrical power, but need not be refilled with helium and can run continuously for an indefinite period. Objects may be cooled by attaching them to a metallic coldplate inside a vacuum ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |