|
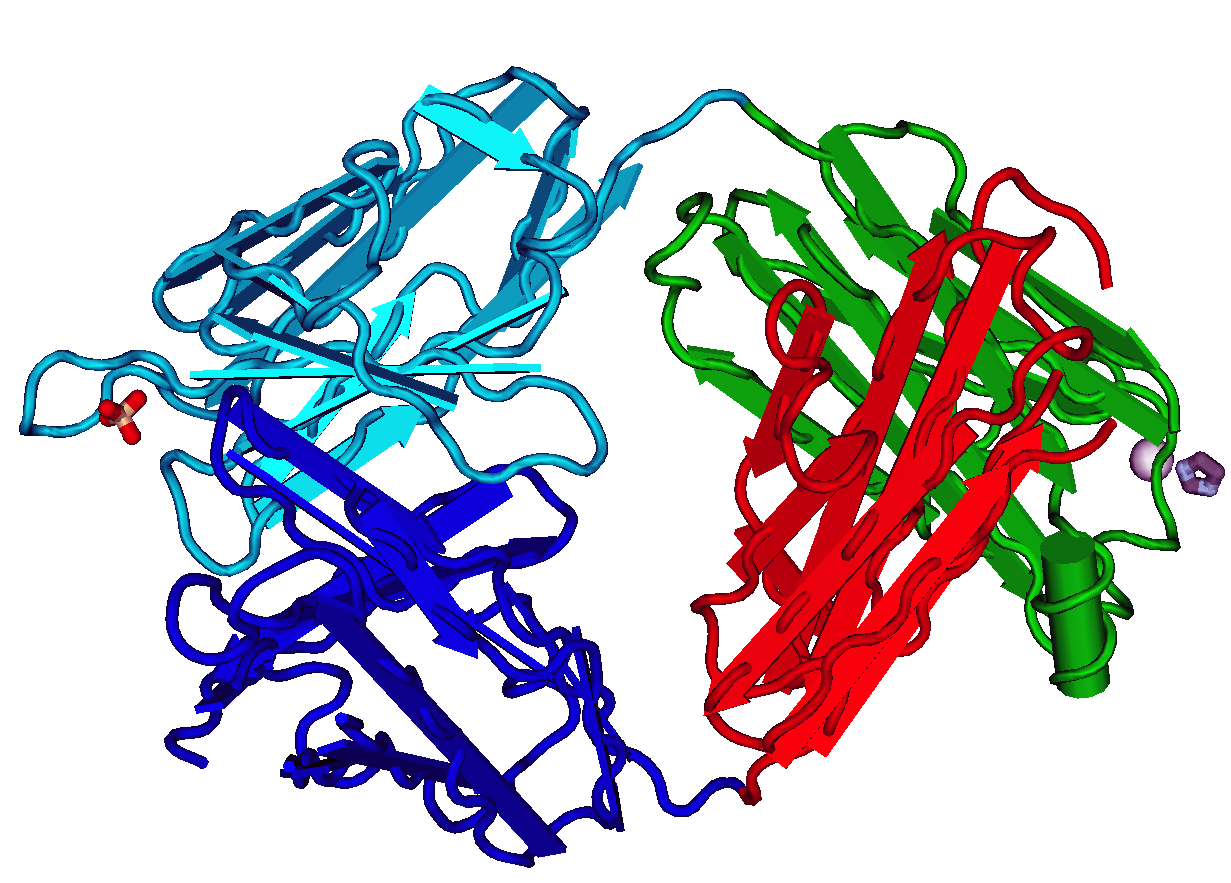
MAb114
Ansuvimab, sold under the brand name Ebanga, is a monoclonal antibody medication for the treatment of ''Zaire ebolavirus'' (Ebolavirus) infection. The most common symptoms include fever, tachycardia (fast heart rate), diarrhea, vomiting, hypotension (low blood pressure), tachypnea (fast breathing) and chills; however, these are also common symptoms of Ebolavirus infection. Ansuvimab was approved for medical use in the United States in December 2020. Chemistry The drug is composed of a single monoclonal antibody (mAb) and was initially isolated from immortalized B-cells that were obtained from a survivor of the 1995 outbreak of Ebola virus disease in Kikwit, Democratic Republic of Congo. In work supported by the United States National Institutes of Health and the Defense Advanced Projects Agency, the heavy and light chain sequences of ansuvimab mAb was cloned into CHO cell lines and initial production runs were produced by Cook Phamica d.b.a. Catalent under contract of Medi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vaccine Research Center
The Vaccine Research Center (VRC), is an intramural division of the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), US Department of Health and Human Services (HHS). The mission of the VRC is to discover and develop both vaccines and antibody-based products that target infectious diseases. The broad research portfolio of the VRC includes basic, clinical, and translational research into vaccines for HIV, Ebola, Marburg, and RSV, among other viruses, and therapeutic antibodies against SARS-CoV-2 (the virus responsible for COVID-19) and other pathogens. History The origins of the Vaccine Research Center date back to 1996 following discussions between President Bill Clinton and NIAID Director Dr. Anthony Fauci regarding research addressing HIV/AIDS. Recognizing the potential impact a vaccine could make in decreasing the global public health burden of HIV, President Clinton in 1997 announced a plan to establish an HIV ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nancy Sullivan (biologist)
Nancy Jean Sullivan is an American cell biologist researching filovirus immunology and vaccine development. She is a senior investigator and chief of the biodefense research section at the Vaccine Research Center. Her team discovered the monoclonal antibody, mAb114. Education Sullivan completed a doctor of science from Harvard T.H. Chan School of Public Health in 1997. She conducted a dissertation in the laboratory of Joseph Sodroski, where her work demonstrated that primary HIV isolates exhibit resistance to antibody neutralization due to occlusion of the coreceptor binding site on gp120. Following her work on HIV, Sullivan pursued postdoctoral training under the guidance of Gary Nabel, studying the mechanisms of Ebola virus pathogenesis and immune protection. Career Sullivan is a cell biologist. She is a tenured senior investigator and chief of the biodefense research section at the Vaccine Research Center The Vaccine Research Center (VRC), is an intramural division of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Julie Ledgerwood
Julie E. Ledgerwood is an American allergist and immunologist, who is the chief medical officer and serves as chief of the Clinical Trials Program at the Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health in Bethesda, Maryland. She is a Doctor of Osteopathic Medicine. Ledgerwood leads clinical trials and clinical collaborations for the VRC; and has served as principal investigator, protocol chair, or associate investigator for over 60 Phase 1-2b clinical trials studying vaccines and monoclonal antibodies targeting pathogens such as HIV, influenza, Ebola, malaria, Chikungunya, and Zika in over 13 countries. She led the first human trial aimed at testing a vaccine for Ebola virus and the first evaluation of mAb114, a monoclonal antibody targeting Ebola. For the past 15 years, she has conducted research with numerous academic research teams and has led international vaccine research collabor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jean-Jacques Muyembe-Tamfum
Jean-Jacques Muyembe is a Congolese microbiologist. He is the general director of the Democratic Republic of the Congo Institut National pour la Recherche Biomedicale (''INRB''). He was part of team at the Yambuku Catholic Mission Hospital that investigated the first Ebola outbreak, and was part of the effort that discovered Ebola as a new disease, although his exact role is still subject to controversy. In 2016, he led the research that designed, along with other researchers at the INRB and the National Institute of Health Vaccine Research Center in the US, one of the most promising treatment for Ebola, mAb114. The treatment was successfully experimented during recent outbreaks in the DRC, on the express decision of the then DRC Minister of Health, Dr Oly Ilunga, despite a prior negative advice from the World Health Organization. Early life and education Muyembe grew up in Bandundu Province, the child of farmers. He was educated in schools run by Jesuits. He studied medicin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fragment Antigen-binding
The fragment antigen-binding region (Fab region) is a region on an antibody that binds to antigens. It is composed of one constant and one variable domain of each of the heavy and the light chain. The variable domain contains the paratope (the antigen-binding site), comprising a set of complementarity-determining regions, at the amino terminal end of the monomer. Each arm of the Y thus binds an epitope on the antigen. Preparation In an experimental setting, Fc and Fab fragments can be generated in the laboratory. The enzyme papain can be used to cleave an immunoglobulin monomer into two Fab fragments and an Fc fragment. Conversely, the enzyme pepsin cleaves below the hinge region, so the result instead is a F(ab')2 fragment and a pFc' fragment. Recently another enzyme for generation of F(ab')2 has been commercially available. The enzyme IdeS (Immunoglobulin degrading enzyme from ''Streptococcus pyogenes'', trade name FabRICATOR) cleaves IgG in a sequence specific manner at neut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase 1 Clinical Trial
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Summary Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory authority for use in the general population. Phase IV trials are 'post-marketing' or 'surveillance' studies conducted to monitor safety over severa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States Army Medical Research Institute Of Infectious Diseases
The United States Army Medical Research Institute of Infectious Diseases (USAMRIID; pronounced: you-SAM-rid) is the U.S Army's main institution and facility for defensive research into countermeasures against biological warfare. It is located on Fort Detrick, Maryland, near Washington, D.C. and is a subordinate lab of the U.S. Army Medical Research and Development Command (USAMRDC), headquartered on the same installation. USAMRIID is the only U.S. Department of Defense (DoD) laboratory equipped to study highly hazardous viruses at Biosafety Level 4 within positive pressure personnel suits. USAMRIID employs both military and civilian scientists as well as highly specialized support personnel, totaling around 800 people. In the 1950s and '60s, USAMRIID and its predecessor unit pioneered unique, state-of-the-art biocontainment facilities which it continues to maintain and upgrade. Investigators at its facilities frequently collaborate with the Centers for Disease Control and Pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Institut National Pour La Recherche Biomedicale
The Institut National de la Recherche Biomédicale (INRB) is the national medical research organization of the Democratic Republic of the Congo. The responsible ministry is the Ministry of Scientific Research and Technology. The National Biomedical Research Institute (INRB) was founded in 1984, it is a 70,000 m² establishment. It has been a collaborating center of the World Health Organization since 2018, headed by Professor Jean-Jacques Muyembe-Tamfum, MD, Ph., Which serves as a national biomedical research laboratory for the Ministry of Health of the Democratic Republic of Congo (DRC) ). It is a multidisciplinary institute which collectively has hundreds of years of experience both in the identification, treatment and prevention of diseases in the DRC. Its foundations are the performance of medical and biological analyzes, applied and translational research, the surveillance of communicable diseases and the promotion of professional growth and development. The INRB has continuo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complement System
The complement system, also known as complement cascade, is a part of the immune system that enhances (complements) the ability of antibodies and phagocytic cells to clear microbes and damaged cells from an organism, promote inflammation, and attack the pathogen's cell membrane. It is part of the innate immune system, which is not adaptable and does not change during an individual's lifetime. The complement system can, however, be recruited and brought into action by antibodies generated by the adaptive immune system. The complement system consists of a number of small proteins that are synthesized by the liver, and circulate in the blood as inactive precursors. When stimulated by one of several triggers, proteases in the system cleave specific proteins to release cytokines and initiate an amplifying cascade of further cleavages. The end result of this ''complement activation'' or ''complement fixation'' cascade is stimulation of phagocytes to clear foreign and damaged material ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phagocytosis
Phagocytosis () is the process by which a cell uses its plasma membrane to engulf a large particle (≥ 0.5 μm), giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is called a phagocyte. In a multicellular organism's immune system, phagocytosis is a major mechanism used to remove pathogens and cell debris. The ingested material is then digested in the phagosome. Bacteria, dead tissue cells, and small mineral particles are all examples of objects that may be phagocytized. Some protozoa use phagocytosis as means to obtain nutrients. History Phagocytosis was first noted by Canadian physician William Osler (1876), and later studied and named by Élie Metchnikoff (1880, 1883). In immune system Phagocytosis is one main mechanisms of the innate immune defense. It is one of the first processes responding to infection, and is also one of the initiating branches of an adaptive immune response. Although mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibody-dependent Cellular Cytotoxicity
Antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system actively lyses a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection. ADCC is independent of the immune complement system that also lyses targets but does not require any other cell. ADCC requires an effector cell which classically is known to be natural killer (NK) cells that typically interact with immunoglobulin G (IgG) antibodies. However, macrophages, neutrophils and eosinophils can also mediate ADCC, such as eosinophils killing certain parasitic worms known as helminths via IgE antibodies. In general, ADCC has typically been described as the immune response to antibody-coated cells leading ultimatel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)
