|
Molybdenum(II) Iodide
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm. Molybdenum does not occur naturally as a free metal on Earth; it is found only in various oxidation states in minerals. The free element, a silvery metal with a grey cast, has the sixth-highest melting point of any element. It readily forms hard, stable carbides in alloys, and for this reason most of the world production of the element (about 80%) is used in steel alloys, including high-strength alloys and superalloys. Most molybdenum compounds have low solubi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler substances by any chemical reaction. The number of protons in the nucleus is the defining property of an element, and is referred to as its atomic number (represented by the symbol ''Z'') – all atoms with the same atomic number are atoms of the same element. Almost all of the baryonic matter of the universe is composed of chemical elements (among rare exceptions are neutron stars). When different elements undergo chemical reactions, atoms are rearranged into new compounds held together by chemical bonds. Only a minority of elements, such as silver and gold, are found uncombined as relatively pure native element minerals. Nearly all other naturally occurring elements occur in the Earth as compounds or mixtures. Air is primarily a mixture o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be " miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Physics'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Essential Element
In the context of nutrition, a mineral is a chemical element required as an essential nutrient by organisms to perform functions necessary for life. However, the four major structural elements in the human body by weight (oxygen, hydrogen, carbon, and nitrogen), are usually not included in lists of major nutrient minerals (nitrogen is considered a "mineral" for plants, as it often is included in fertilizers). These four elements compose about 96% of the weight of the human body, and major minerals (macrominerals) and minor minerals (also called trace elements) compose the remainder. Nutrient minerals, being elements, cannot be synthesized biochemically by living organisms. Plants get minerals from soil. Most of the minerals in a human diet come from eating plants and animals or from drinking water. As a group, ''minerals'' are one of the four groups of essential nutrients, the others of which are vitamins, essential fatty acids, and essential amino acids. The five major minerals i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdopterin
Molybdopterins are a class of cofactors found in most molybdenum-containing and all tungsten-containing enzymes. Synonyms for molybdopterin are: MPT and pyranopterin-dithiolate. The nomenclature for this biomolecule can be confusing: Molybdopterin itself contains no molybdenum; rather, this is the name of the ligand (a ''pterin'') that will bind the active metal. After molybdopterin is eventually complexed with molybdenum, the complete ligand is usually called molybdenum cofactor. Molybdopterin consists of a pyranopterin, a complex heterocycle featuring a pyran fused to a pterin ring. In addition, the pyran ring features two thiolates, which serve as ligands in molybdo- and tungstoenzymes. In some cases, the alkyl phosphate group is replaced by an alkyl diphosphate nucleotide. Enzymes that contain the molybdopterin cofactor include xanthine oxidase, DMSO reductase, sulfite oxidase, and nitrate reductase. The only molybdenum-containing enzymes that do not feature molybdopteri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
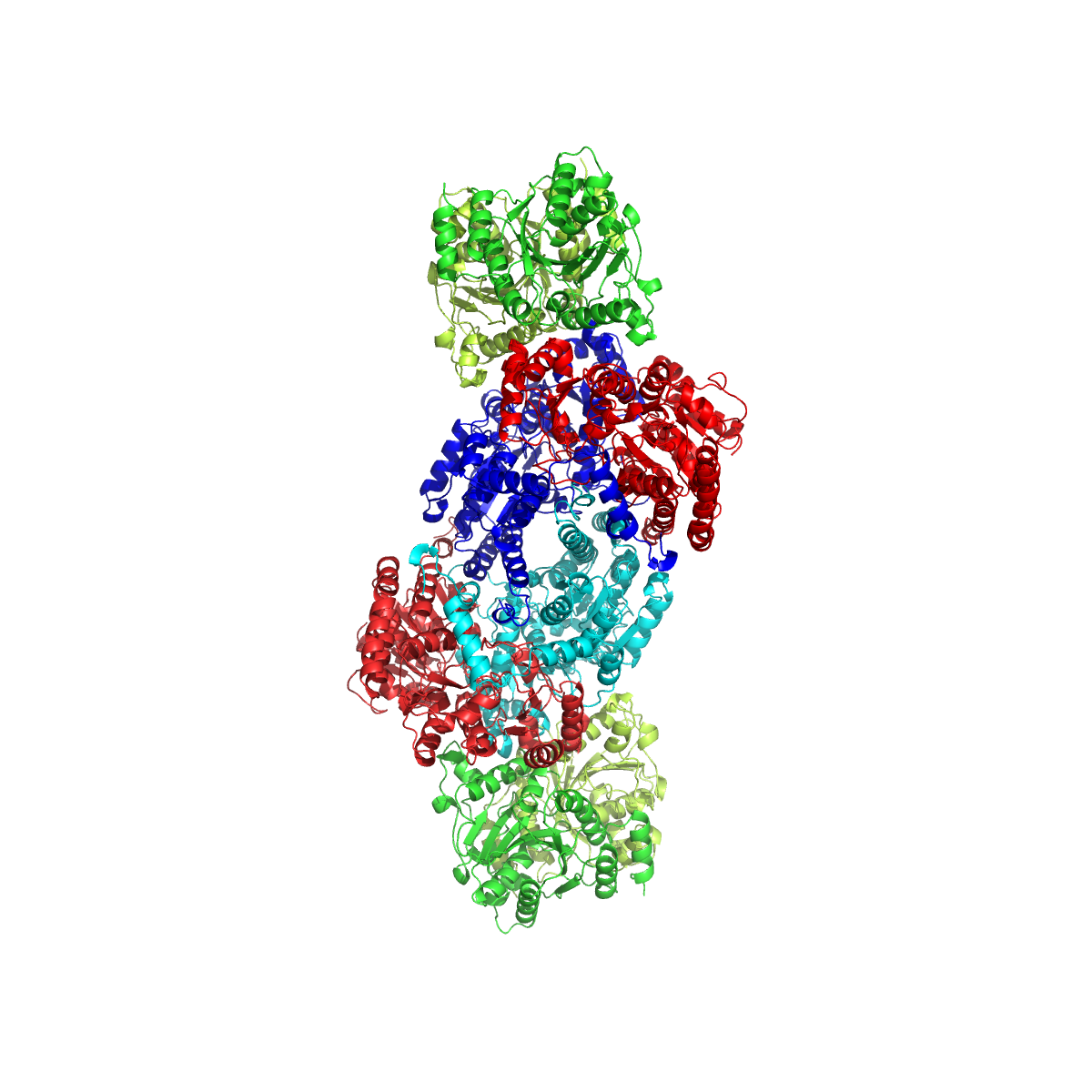
FeMoco
FeMoco ( cofactor) is the primary cofactor of nitrogenase. Nitrogenase is the enzyme that catalyzes the conversion of atmospheric nitrogen molecules N2 into ammonia (NH3) through the process known as nitrogen fixation. Containing iron and molybdenum, the cofactor is called FeMoco. Its stoichiometry is Fe7MoS9C. Structure The FeMo cofactor is a cluster with composition Fe7MoS9C. Fe is the chemical symbol for the element iron (ferrum), and Mo is the symbol for molybdenum. This large cluster can be viewed as two subunits composed of one Fe4S3 (iron(III) sulfide) cluster and one MoFe3S3 cluster. The two clusters are linked by three sulfide ligands. The unique iron (Fe) is anchored to the protein by a cysteine. It is also bound to three sulfides, resulting in tetrahedral molecular geometry. The additional six Fe centers in the cluster are each bonded to three sulfides. These six internal Fe centers define a trigonal prismatic arrangement around a central carbide center. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogenase
Nitrogenases are enzymes () that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the Organic redox reaction, reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only family of enzymes known to catalyze this reaction, which is a key step in the process of nitrogen fixation. Nitrogen fixation is required for all forms of life, with nitrogen being essential for the biosynthesis of molecules (nucleotides, amino acids) that create plants, animals and other organisms. They are encoded by the Nif genes or Homologous chromosome, homologs. They are related to protochlorophyllide reductase. Classification and structure Although the equilibrium formation of ammonia from molecular hydrogen and nitrogen has an overall negative enthalpy of reaction ( \Delta H^ = -45.2 \ \mathrm \, \mathrm \; \mathrm ), the activation energy is very high ( E_\mathrm = 230-420 \ \mathrm \, \mathrm ). Nitrogenase a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Fixation
Nitrogen fixation is a chemical process by which molecular nitrogen (), with a strong triple covalent bond, in the air is converted into ammonia () or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. Atmospheric nitrogen is molecular dinitrogen, a relatively nonreactive molecule that is metabolically useless to all but a few microorganisms. Biological nitrogen fixation or ''diazotrophy'' is an important microbials mediated process that converts dinitrogen (N2) gas to ammonia (NH3) using the nitrogenase protein complex (Nif). Nitrogen fixation is essential to life because fixed inorganic nitrogen compounds are required for the biosynthesis of all nitrogen-containing organic compounds, such as amino acids and proteins, nucleoside triphosphates and nucleic acids. As part of the nitrogen cycle, it is essential for agriculture and the manufacture of fertilizer. It is also, indirectly, relevant to the manufacture of all nitrogen chemical c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many indus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. The strength of chemical bonds varies considerably; there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force and hydrogen bonding. Strong chemical bonding arises from the sharing or transfer of electrons between the participating atoms. Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compounds. Pigments of prehistoric and historic value include ochre, charcoal, and lapis lazuli. Economic impact In 2006, around 7.4 million tons of inorganic, organic, and special pigments were marketed worldwide. Estimated at around US$14.86 billion in 2018 and will rise at over 4.9% CAGR from 2019 to 2026. The global demand for pigments was roughly US$20.5 billion in 2009. According to an April 2018 report by ''Bloomberg Businessweek'', the estimated value of the pigment industry globally is $30 billion. The value of titanium dioxide – used to enhance the white brightness of many products – was placed at $13.2 billion per year, while the color Ferrari red is valued at $300 million each year. Physical principles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High-pressure
In science and engineering the study of high pressure examines its effects on materials and the design and construction of devices, such as a diamond anvil cell, which can create high pressure. By ''high pressure'' is usually meant pressures of thousands (kilo bars) or millions (megabars) of times atmospheric pressure (about 1 bar or 100,000 Pa). History and overview Percy Williams Bridgman received a Nobel Prize in 1946 for advancing this area of physics by several magnitudes of pressure (400 MPa to 40,000 MPa). The list of founding fathers of this field includes also the names of Harry George Drickamer, Tracy Hall, Francis P. Bundy, Leonid F. Vereschagin, and Sergey M. Stishov. It was by applying high pressure as well as high temperature to carbon that man-made diamonds were first produced as well as many other interesting discoveries. Almost any material when subjected to high pressure will compact itself into a denser form, for example, quartz, also called silica or silicon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

%2C_black_and_white.png)