|
Metacaspase
Metacaspases are members of the C14 class of cysteine proteases and thus related to caspases, orthocaspases and paracaspases. The metacaspases are arginine/lysine-specific, in contrast to caspases, which are aspartate-specific. Structure and Phylogenetic distribution Prokaryotes In archea and bacteria, there are several metacaspases with a wide range of domain organizations. Based on the prokaryote metacaspase diversity, orthocaspases can be considered a sub-class of metacaspases. Common for both metacaspases and orthocaspases classes is their specificity for basic residues (arginine or lysine) in the P1 position. At this moment, no structural variants have been reported where the substrate specificity would change to an acidic residue (aspartic acid), like in true caspases. Eukaryotes Metacaspases are found in plants, fungi, and "protists", but not in slime mold or animals. Viruses Viral metacaspases, which may have implications in rewiring host metabolism to enhance infe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paracaspase
Paracaspases (human: MALT1) are members of the C14 family of cysteine proteases. Paracaspases are proteins related to caspases present in animals and slime mold, in contrast to metacaspases, which are present in plants, fungi, and "protists". The phylogenetic distribution is a bit confusing, since slime mold diverged earlier than the animal/fungal split. Paracaspase has been first identified in a recurrent t(11;18)(q21;q21) chromosomal translocation associated with a subset of MALT lymphoma. This leads to a fusion oncoprotein consisting of the carboxyl terminus of MALT1 and the amino terminus of BIRC3, c-IAP2. Paracaspases are more similar to caspases than metacaspases are, indicating that this group of proteases diverged from caspases from a common metacaspase ancestor. Structure and Evolution Most non-metazoan paracaspases found in amoebas or bacteria are "type 2" paracaspases with only a caspase-like domain. The animal paracaspases are most likely not directly related to the a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
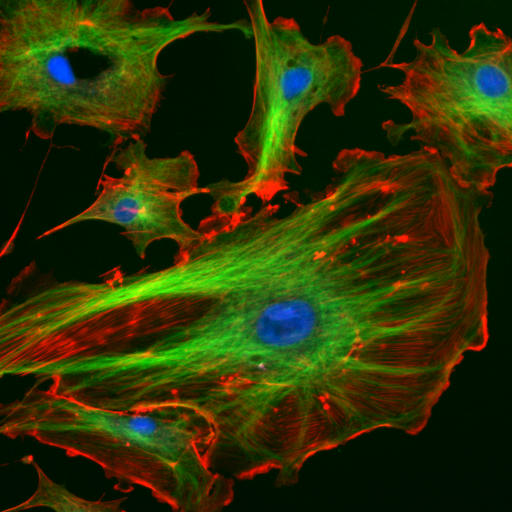
Caspase
Caspases (cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate-directed proteases) are a family of protease enzymes playing essential roles in programmed cell death. They are named caspases due to their specific cysteine protease activity – a cysteine in its active site nucleophilically attacks and cleaves a target protein only after an aspartic acid residue. As of 2009, there are 12 confirmed caspases in humans and 10 in mice, carrying out a variety of cellular functions. The role of these enzymes in programmed cell death was first identified in 1993, with their functions in apoptosis well characterised. This is a form of programmed cell death, occurring widely during development, and throughout life to maintain cell homeostasis. Activation of caspases ensures that the cellular components are degraded in a controlled manner, carrying out cell death with minimal effect on surrounding tissues. Caspases have other identified roles in programmed cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine Protease
Cysteine proteases, also known as thiol proteases, are hydrolase enzymes that degrade proteins. These proteases share a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic triad or dyad. Discovered by Gopal Chunder Roy in 1873, the first cysteine protease to be isolated and characterized was papain, obtained from ''Carica papaya''. Cysteine proteases are commonly encountered in fruits including the papaya, pineapple, fig and kiwifruit. The proportion of protease tends to be higher when the fruit is unripe. In fact, the latex of dozens of different plant families are known to contain cysteine proteases. Cysteine proteases are used as an ingredient in meat tenderizers. Classification The MEROPS protease classification system counts 14 superfamilies plus several currently unassigned families (as of 2013) each containing many families. Each superfamily uses the catalytic triad or dyad in a different protein fold and so represent convergent evo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral, polar amino acid. It is non-essential and conditionally essential in humans, meaning the body can usually synthesize sufficient amounts of it, but in some instances of stress, the body's demand for glutamine increases, and glutamine must be obtained from the diet. It is encoded by the codons CAA and CAG. It is named after glutamic acid, which in turn is named after its discovery in cereal proteins, gluten. In human blood, glutamine is the most abundant free amino acid. The dietary sources of glutamine include especially the protein-rich foods like beef, chicken, fish, dairy products, eggs, vegetables like beans, beets, cabbage, spinach, carrots, parsley, vegetable juices and also in wheat, papaya, Brussels sprouts, celery, kale ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eukaryote Proteins
The eukaryotes ( ) constitute the domain of Eukaryota or Eukarya, organisms whose cells have a membrane-bound nucleus. All animals, plants, fungi, seaweeds, and many unicellular organisms are eukaryotes. They constitute a major group of life forms alongside the two groups of prokaryotes: the Bacteria and the Archaea. Eukaryotes represent a small minority of the number of organisms, but given their generally much larger size, their collective global biomass is much larger than that of prokaryotes. The eukaryotes emerged within the archaeal kingdom Promethearchaeati and its sole phylum Promethearchaeota. This implies that there are only two domains of life, Bacteria and Archaea, with eukaryotes incorporated among the Archaea. Eukaryotes first emerged during the Paleoproterozoic, likely as flagellated cells. The leading evolutionary theory is they were created by symbiogenesis between an anaerobic Promethearchaeati archaean and an aerobic proteobacterium, which formed the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Programmed Cell Death
Programmed cell death (PCD) sometimes referred to as cell, or cellular suicide is the death of a cell (biology), cell as a result of events inside of a cell, such as apoptosis or autophagy. PCD is carried out in a biological process, which usually confers advantage during an organism's biological life cycle, lifecycle. For example, the Limb development, differentiation of fingers and toes in a developing human embryo occurs because cells between the fingers apoptose; the result is that the digits are separate. PCD serves fundamental functions during both plant and animal tissue development. Apoptosis and autophagy are both forms of programmed cell death. Necrosis is the death of a cell caused by external factors such as trauma or infection and occurs in several different forms. Necrosis was long seen as a non-physiological process that occurs as a result of infection or injury, but in the 2000s, a form of programmed necrosis, called necroptosis, was recognized as an alternative f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MALT1
Mucosa-associated lymphoid tissue lymphoma translocation protein 1 is a protein that in humans is encoded by the ''MALT1'' gene. It's the human paracaspase. Function Genetic ablation of the paracaspase gene in mice and biochemical studies have shown that paracaspase is a crucial protein for T and B lymphocytes activation. It has an important role in the activation of the transcription factor NF-κB, in the production of interleukin-2 (IL-2) and in T and B lymphocytes proliferation Two alternatively spliced transcript variants encoding different isoforms have been described for this gene. In addition, a role for paracaspase has been shown in the innate immune response mediated by the zymosan receptor Dectin-1 in macrophages and dendritic cells, and in response to the stimulation of certain G protein-coupled receptors. Sequence analysis proposes that paracaspase has an N-terminal death domain, two central immunoglobulin-like domains involved in the binding to the B-cell lymph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Proteolysis Map
The Proteolysis MAP (PMAP) was an integrated web resource focused on proteases. Its domain now links to a scam/spam browser extender. Rationale PMAP was designed to aid the protease researchers in reasoning about proteolytic networks and metabolic pathways. History and funding PMAP was originally created at the Burnham Institute for Medical Research, La Jolla, California. In 2004 the National Institutes of Health (NIH) selected a team led by Jeffrey W. Smith, to establish the Center on Proteolytic Pathways (CPP). As part of the NIH Roadmap for Biomedical research, the center develops technology to study the behavior of proteins and to disburse that knowledge to the scientific community at large. Focal point Proteases are a class of enzymes that regulate much of what happens in the human body, both inside the cell and out, by cleaving peptide bonds in proteins. Through this activity, they govern the four essential cell functions: differentiation, motility, division and cel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom (biology), kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constitute 1% of all described fungal species. Some yeast species have the ability to develop multicellular characteristics by forming strings of connected budding cells known as pseudohyphae or false hyphae, or quickly evolve into a Multicellular organism, multicellular cluster with specialised Organelle, cell organelles function. Yeast sizes vary greatly, depending on species and environment, typically measuring 3–4 micrometre, μm in diameter, although some yeasts can grow to 40 μm in size. Most yeasts reproduce asexual reproduction, asexually by mitosis, and many do so by the asymmetric division process known as budding. With their single-celled growth habit, yeasts can be contrasted with Mold (fungus), molds, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Programmed Cell Death
Programmed cell death (PCD) sometimes referred to as cell, or cellular suicide is the death of a cell (biology), cell as a result of events inside of a cell, such as apoptosis or autophagy. PCD is carried out in a biological process, which usually confers advantage during an organism's biological life cycle, lifecycle. For example, the Limb development, differentiation of fingers and toes in a developing human embryo occurs because cells between the fingers apoptose; the result is that the digits are separate. PCD serves fundamental functions during both plant and animal tissue development. Apoptosis and autophagy are both forms of programmed cell death. Necrosis is the death of a cell caused by external factors such as trauma or infection and occurs in several different forms. Necrosis was long seen as a non-physiological process that occurs as a result of infection or injury, but in the 2000s, a form of programmed necrosis, called necroptosis, was recognized as an alternative f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eukaryotes
The eukaryotes ( ) constitute the domain of Eukaryota or Eukarya, organisms whose cells have a membrane-bound nucleus. All animals, plants, fungi, seaweeds, and many unicellular organisms are eukaryotes. They constitute a major group of life forms alongside the two groups of prokaryotes: the Bacteria and the Archaea. Eukaryotes represent a small minority of the number of organisms, but given their generally much larger size, their collective global biomass is much larger than that of prokaryotes. The eukaryotes emerged within the archaeal kingdom Promethearchaeati and its sole phylum Promethearchaeota. This implies that there are only two domains of life, Bacteria and Archaea, with eukaryotes incorporated among the Archaea. Eukaryotes first emerged during the Paleoproterozoic, likely as flagellated cells. The leading evolutionary theory is they were created by symbiogenesis between an anaerobic Promethearchaeati archaean and an aerobic proteobacterium, which form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Horizontal Gene Transfer
Horizontal gene transfer (HGT) or lateral gene transfer (LGT) is the movement of genetic material between organisms other than by the ("vertical") transmission of DNA from parent to offspring (reproduction). HGT is an important factor in the evolution of many organisms. HGT is influencing scientific understanding of higher-order evolution while more significantly shifting perspectives on bacterial evolution. Horizontal gene transfer is the primary mechanism for the spread of antibiotic resistance in bacteria, and plays an important role in the evolution of bacteria that can degrade novel compounds such as human-created Bactericide, pesticides and in the evolution, maintenance, and transmission of virulence. It often involves Temperateness (virology), temperate bacteriophages and plasmids. Genes responsible for antibiotic resistance in one species of bacteria can be transferred to another species of bacteria through various mechanisms of HGT such as Transformation (genetics), tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |