|
Matrix Effects
In chemical analysis, matrix refers to the components of a sample other than the analyte of interest. The matrix can have a considerable effect on the way the analysis is conducted and the quality of the results are obtained; such effects are called matrix effects.F. W. Fifield, P. J. Haines. ''Environmental Analytical Chemistry''. Blackwell Publishing, 2000, p. 4-5. . For example, the ionic strength of the solution can have an effect on the activity coefficients of the analytes.Harris, D. C. ''Quantitative Chemical Analysis'', 4th ed. Freeman, 1995, pp.194, 404. . The most common approach for accounting for matrix effects is to build a calibration curve using standard samples with known analyte concentration and which try to approximate the matrix of the sample as much as possible. This is especially important for solid samples where there is a strong matrix influence.Marco Aurelio Zezzi Arruda. ''Trends in Sample Preparation''. Nova Publishers, 2006, p. 15-18. . In cases with comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Analysis
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration. Analytical chemistry consists of classical, wet chemical methods and modern analytical techniques. Classical qualitative methods use separations such as precipitation, extraction, and distillation. Identification may be based on differences in color, odor, melting point, boiling point, solubility, radioactivity or reactivity. Classical quantitative analysis uses mass or volume changes to quantify amount. Instrumental methods may be used to separate samples using chromatography, electrophoresis or field flow fractionation. Then qualitative and quantitative analysis can be performed, often wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sample (material)
In general, a sample is a limited quantity of something which is intended to be similar to and represent a larger amount of that thing(s). The things could be Count noun, countable objects such as individual items available as units for sale, or an Mass noun, uncountable material. Even though the word "sample" implies a smaller quantity taken from a larger amount, sometimes full Biological specimen, biological or Type specimen (mineralogy), mineralogical specimens are called samples if they are taken for analysis, testing, or investigation like other samples. They are also considered samples in the sense that even whole specimens are "samples" of the full population of many individual organisms. The act of obtaining a sample is called "sampling" and can be performed manually by a person or by automatic process. Samples of material can be taken or provided for testing, Analyser, analysis, investigation, Acceptance sampling, quality control, demonstration, or trial use. Sometime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analyte
An analyte, component (in clinical chemistry), titrand (in titrations), or chemical species is a substance or chemical constituent that is of interest in an analytical procedure. The remainder of the sample is called the matrix. The procedure of analysis measures the analyte's chemical or physical properties, thus establishing its identity or concentration in the sample. See also *Analytical chemistry * Standard solution *Immunoassay An immunoassay (IA) is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody (usually) or an antigen (sometimes). The molecule detected by the immunoassay ... * Magnetic immunoassay References Analytical chemistry {{Analytical-chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation constant or the solubility of different salts. One of the main characteristics of a solution with dissolved ions is the ionic strength. Ionic strength can be molar (mol/L solution) or molal (mol/kg solvent) and to avoid confusion the units should be stated explicitly. The concept of ionic strength was first introduced by Lewis and Randall in 1921 while describing the activity coefficients of strong electrolytes. Quantifying ionic strength The molar ionic strength, ''I'', of a solution is a function of the concentration of ''all'' ions present in that solution. :I = \begin\frac\end\sum_^ c_i z_i^ where one half is because we are including both cations and anions, ''c''i is the molar concentration of ion i (M, mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calibration Curve
In analytical chemistry, a calibration curve, also known as a standard curve, is a general method for determining the concentration of a substance in an unknown sample by comparing the unknown to a set of standard samples of known concentration. A calibration curve is one approach to the problem of instrument calibration; other standard approaches may mix the standard into the unknown, giving an internal standard. The calibration curve is a plot of how the instrumental response, the so-called analytical signal, changes with the concentration of the analyte (the substance to be measured). General use In more general use, a calibration curve is a Graph of a function, curve or Table (information), table for a measuring instrument which measures some parameter indirectly, giving values for the desired quantity as a function of values of sensor output. For example, a calibration curve can be made for a particular pressure transducer to determine applied pressure from transducer output ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
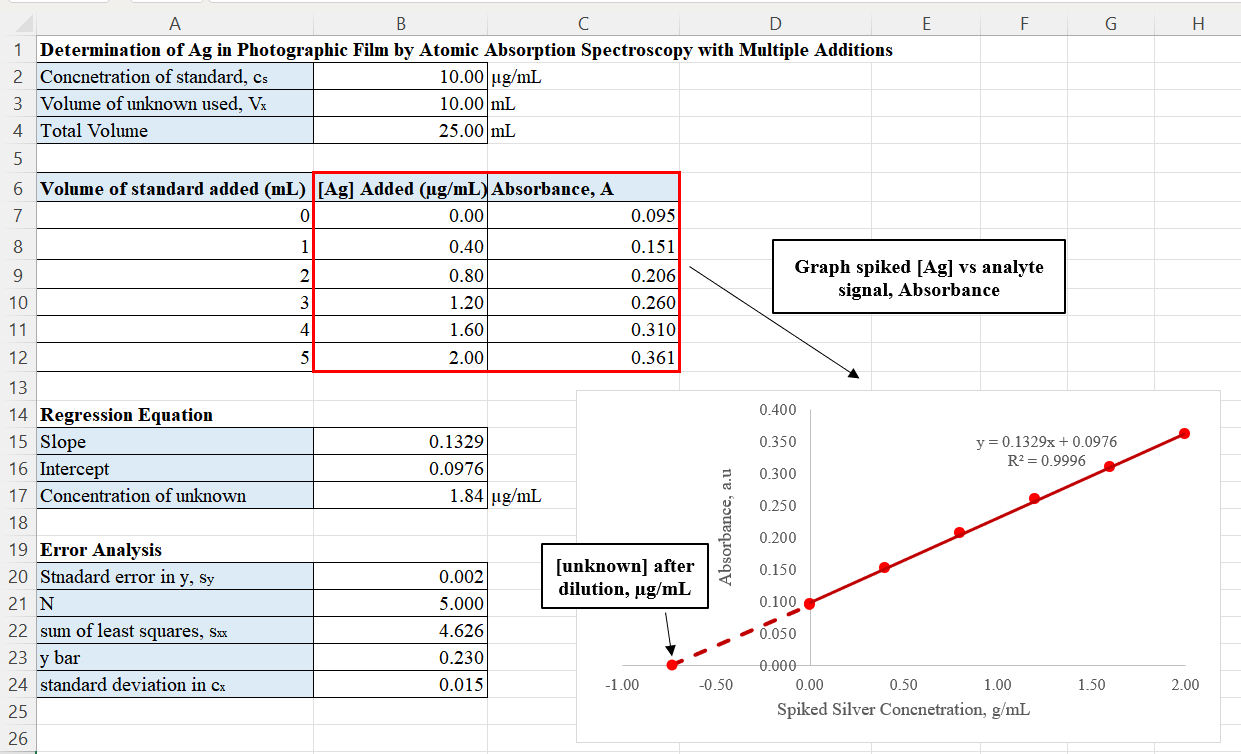
Standard Addition Method
The Standard addition method, also called known addition, often used in analytical chemistry, quantifies the analyte present in an unknown. This method is useful for analyzing complex samples where a matrix effect interferes with the analyte signal. In comparison to the calibration curve method, the standard addition method has the advantage of the matrices of the unknown and standards being nearly identical. This minimizes the potential bias arising from the matrix effect when determining the concentration. Variations Standard addition involves adding known amounts of analyte to an unknown sample, a process known as ''spiking''. By increasing the number of spikes, the analyst can extrapolate for the analyte concentration in the unknown that has not been spiked. There are multiple approaches to the standard addition. The following section summarize each approach. Single standard addition used in polarography In classic polarography, the standard addition method involves creati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a variety of materials (chemicals) depending on the type of cell. An electrode may be called either a cathode or anode according to the direction of the electric current, unrelated to the potential difference between electrodes. Michael Faraday coined the term "" in 1833; the word recalls the Greek ἤλεκτρον (, "amber") and ὁδός (, "path, way"). The electrophore, invented by Johan Wilcke in 1762, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any battery. The first electrochemical battery was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consisted of a stack of copper and zinc electrodes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standard Solution
In analytical chemistry, a standard solution (titrant or titrator) is a solution containing an accurately known concentration. Standard solutions are generally prepared by dissolving a solute of known mass into a solvent to a precise volume, or by diluting a solution of known concentration with more solvent. A standard solution ideally has a high degree of purity and is stable enough that the concentration can be accurately measured after a long shelf time. Making a standard solution requires great attention to detail to avoid introducing any risk of contamination that could diminish the accuracy of the concentration. For this reason, glassware with a high degree of precision such as a volumetric flask, volumetric pipette, micropipettes, and automatic pipettes are used in the preparation steps. The solvent used must also be pure and readily able to dissolve the solute into a homogenous solution. Standard solutions are used for various volumetric procedures, such as determining ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for Separation process, separating and analyzing compounds that can be vaporized without Chemical decomposition, decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture. Gas chromatography is also sometimes known as vapor-phase chromatography (VPC), or gas–liquid partition chromatography (GLPC). These alternative names, as well as their respective abbreviations, are frequently used in scientific literature. Gas chromatography is the process of separating compounds in a mixture by injecting a gaseous or liquid sample into a mobile phase, typically called the carrier gas, and passing the gas through a stationary phase. The mobile phase is usually an inert gas or an Reactivity (chemistry), unreactive gas such as helium, arg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High-performance Liquid Chromatography
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can originate from food, chemicals, pharmaceuticals, biological, environmental and agriculture, etc., which have been dissolved into liquid solutions. It relies on high pressure pumps, which deliver mixtures of various solvents, called the mobile phase, which flows through the system, collecting the sample mixture on the way, delivering it into a cylinder, called the column, filled with solid particles, made of adsorbent material, called the stationary phase. Each component in the sample interacts differently with the adsorbent material, causing different migration rates for each component. These different rates lead to separation as the species flow out of the column into a specific detector such as UV detectors. The output of the detecto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extraction (chemistry)
Extraction in chemistry is a separation process consisting of the separation of a substance from a Matrix (chemical analysis), matrix. The distribution of a solute between two phases is an Chemical equilibrium, equilibrium condition described by partition theory. This is based on exactly how the analyte moves from the initial solvent into the extracting solvent. The term ''washing'' may also be used to refer to an extraction in which impurities are extracted from the solvent containing the desired Chemical compound, compound. Types of extraction * Liquid–liquid extraction ** Acid-base extraction ** Supercritical fluid extraction * Solid-liquid extraction ** Solid-phase extraction ** Maceration ** Ultrasound assisted extraction, Ultrasound-assisted extraction ** Microwave-assisted extraction ** Heat reflux extraction ** Instant controlled pressure drop extraction (:fr:Détente instantanée contrôlée, Détente instantanée contrôlée) * Perstraction Laboratory applica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |