|
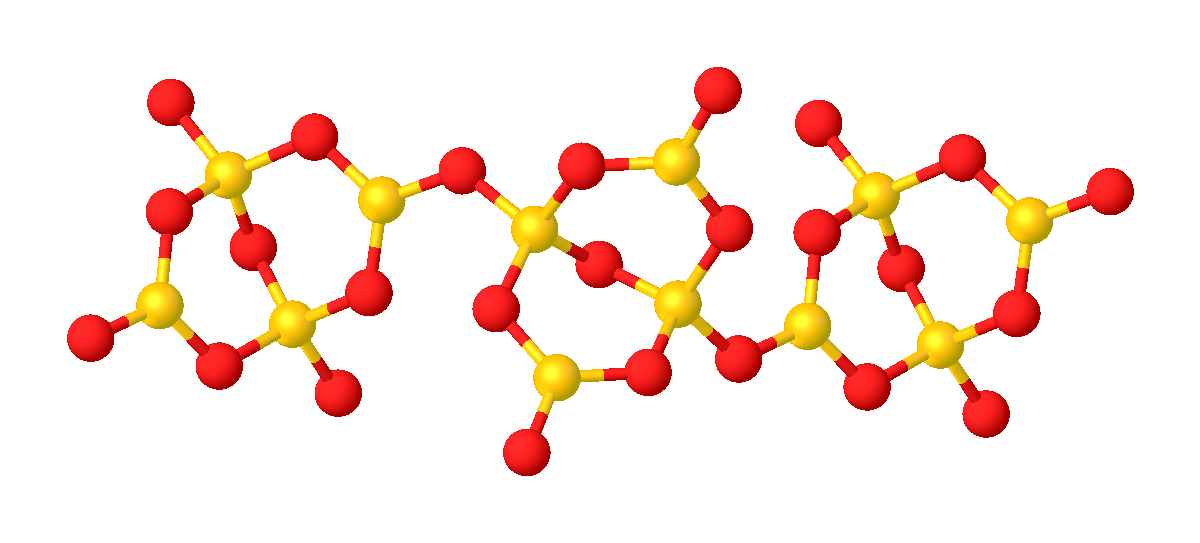
Lithium Tetraborate(6-)
Lithium borate, also known as lithium tetraborate is an inorganic compound with the formula Li2B4O7. A colorless solid, lithium borate is used in making glasses and ceramics. Structure Its structure consists of a polymeric borate backbone. The Li+ centers are bound to four and five oxygen ligands. Boron centers are trigonal and tetrahedral. Lithium borate can be used in the laboratory as LB buffer for gel electrophoresis of DNA and RNA. It is also used in the borax fusion method to vitrify mineral powder specimens for analysis by WDXRF spectroscopy.Ron Jenkins, ''X-Ray Fluorescence Spectrometry, Second Edition'', J. Wiley & Sons Inc., 1999, , p 146-7. See also *LB buffer *Lithium metaborate Lithium metaborate is a chemical compound of lithium, boron, and oxygen with elemental formula . It is often encountered as a hydrate, , where ''n'' is usually 2 or 4. However, these formulas do not describe the actual structure of the solids. ... (LiBO2) References Borates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) of the molten form; some glasses such as volcanic glass are naturally occurring. The most familiar, and historically the oldest, types of manufactured glass are "silicate glasses" based on the chemical compound silica (silicon dioxide, or quartz), the primary constituent of sand. Soda–lime glass, containing around 70% silica, accounts for around 90% of manufactured glass. The term ''glass'', in popular usage, is often used to refer only to this type of material, although silica-free glasses often have desirable properties for applications in modern communications technology. Some objects, such as drinking glasses and eyeglasses, are so commonly made of silicate-based glass that they are simply called by the name of the material. Despite bei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ceramic
A ceramic is any of the various hard, brittle, heat-resistant and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain, and brick. The earliest ceramics made by humans were pottery objects (''pots,'' ''vessels or vases'') or figurines made from clay, either by itself or mixed with other materials like silica, hardened and sintered in fire. Later, ceramics were glazed and fired to create smooth, colored surfaces, decreasing porosity through the use of glassy, amorphous ceramic coatings on top of the crystalline ceramic substrates. Ceramics now include domestic, industrial and building products, as well as a wide range of materials developed for use in advanced ceramic engineering, such as in semiconductors. The word "'' ceramic''" comes from the Greek word (), "of pottery" or "for pottery", from (), "potter's clay, tile, pottery". The earliest kno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LB Buffer
LB buffer, also known as lithium borate buffer, is a buffer solution used in agarose electrophoresis, typically for the separation of nucleic acids such as DNA and RNA. It is made up of Lithium borate (lithium hydroxide monohydrate and boric acid). LB(R) is a registered (USPTO) trademark of Faster Better Media LLC, which owns US patent 7,163,610 covering low-conductance lithium borate polynucleotide electrophoresis. Lithium Borate buffer has a lower conductivity, produces crisper resolution, and can be run at higher speeds than can gels made from TBE or TAE (5-50 V/cm as compared to 5-10 V/cm). At a given voltage, the heat generation and thus the gel temperature is much lower than with TBE/TAE buffers, therefore the voltage can be increased to speed up electrophoresis so that a gel run takes only a fraction of the usual time. Downstream applications, such as isolation of DNA from a gel slice or Southern blot analysis, work as expected with lithium boric acid gels. Brody, J.R. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gel Electrophoresis
Gel electrophoresis is a method for separation and analysis of biomacromolecules ( DNA, RNA, proteins, etc.) and their fragments, based on their size and charge. It is used in clinical chemistry to separate proteins by charge or size (IEF agarose, essentially size independent) and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge. Nucleic acid molecules are separated by applying an electric field to move the negatively charged molecules through a matrix of agarose or other substances. Shorter molecules move faster and migrate farther than longer ones because shorter molecules migrate more easily through the pores of the gel. This phenomenon is called sieving. Proteins are separated by the charge in agarose because the pores of the gel are too small to sieve proteins. Gel electrophoresis can also be used for the separation of nanoparticles. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitrification
Vitrification (from Latin ''vitreum'', "glass" via French ''vitrifier'') is the full or partial transformation of a substance into a glass, that is to say, a non-crystalline amorphous solid. Glasses differ from liquids structurally and glasses possess a higher degree of connectivity with the same Hausdorff dimensionality of bonds as crystals: dimH = 3. In the production of ceramics, vitrification is responsible for its impermeability to water. Vitrification is usually achieved by heating materials until they liquidize, then cooling the liquid, often rapidly, so that it passes through the glass transition to form a glassy solid. Certain chemical reactions also result in glasses. In terms of chemistry, vitrification is characteristic for amorphous materials or disordered systems and occurs when bonding between elementary particles (atoms, molecules, forming blocks) becomes higher than a certain threshold value. Thermal fluctuations break the bonds; therefore, the low ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Fluorescence
X-ray fluorescence (XRF) is the emission of characteristic "secondary" (or fluorescent) X-rays from a material that has been excited by being bombarded with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and analytical chemistry, chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science, archaeology and art objects such as paintings. Underlying physics When materials are exposed to short-wavelength X-rays or to gamma rays, ionization of their component atoms may take place. Ionization consists of the ejection of one or more electrons from the atom, and may occur if the atom is exposed to radiation with an energy greater than its ionization energy. X-rays and gamma rays can be energetic enough to expel tightly held electrons from the inner atomic orbital, orbitals of the atom. The removal of an electron in this way makes the electronic structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Metaborate
Lithium metaborate is a chemical compound of lithium, boron, and oxygen with elemental formula . It is often encountered as a hydrate, , where ''n'' is usually 2 or 4. However, these formulas do not describe the actual structure of the solids. Lithium metaborate is one of the borates, a large family of salts (ionic compounds) with anions consisting of boron, oxygen, and hydrogen. Structure Lithium metaborate has several crystal forms. The γ form is stable at 15 kbar and 950 °C. It has a polymeric cation consisting of a tridimensional regular array of tetrahedra sharing oxygen vertices, alernating with lithium cations, each also surrounded by four oxygen atoms. The B-O distances are 148.3 pm, the Li-O distances are 196 pm. Applications Laboratory Molten lithium metaborate, often mixed with lithium tetraborate , is used to dissolve oxide samples for analysis by XRF, AAS, ICP-OES, ICP-AES, and ICP-MS, modern versions of classical bead test. The process may be used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borates
A borate is any of several boron oxyanions, negative ions consisting of boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt with such anions, such as sodium metaborate, and disodium tetraborate . The name also refers to certain functional groups in molecules consisting of boron and oxygen, and esters with such groups, such as triethyl orthoborate . Natural occurrence Borate ions occur, alone or with other anions, in many borate and borosilicate minerals such as borax, boracite, ulexite (boronatrocalcite) and colemanite. Borates also occur in seawater, where they make an important contribution to the absorption of low frequency sound in seawater. Borates also occur in plants, including almost all fruits. Anions The main borate anions are: * tetrahydroxyborate , found in sodium tetrahydroxyborate . * orthoborate , found in trisodium orthoborate * perborate , as in sodium perborate * metaborate or , found in sodium metaborate * diborate , f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |