|
Lapaquistat
Lapaquistat (TAK-475) is a cholesterol-lowering drug candidate that was abandoned before being marketed. Unlike statins, which inhibit HMG-CoA reductase, lapaquistat metabolites inhibit squalene synthase, which is further downstream in the synthesis of cholesterol. It is hoped that side effects can be reduced by not disturbing the mevalonate pathway, which is important for other biochemical molecules besides cholesterol. However, there is increasing evidence that statins (which inhibit the mevalonate pathway) may be clinically useful ''because'' they affect these other molecules (including protein prenylation). On March 28, 2008, Takeda halted further development of lapaquistat. While effective at lowering low-density lipoprotein cholesterol in a dose-dependent manner, development of the drug was ceased due to observations in clinical trials that it might cause liver damage in the high dose trial groups. Data from knockout mouse studies suggests that accumulation of high levels ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Squalene Synthase
Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme localized to the membrane of the endoplasmic reticulum. SQS participates in the isoprenoid biosynthetic pathway, catalyzing a two-step reaction in which two identical molecules of farnesyl pyrophosphate (FPP) are converted into squalene, with the consumption of NADPH. Catalysis by SQS is the first committed step in sterol synthesis, since the squalene produced is converted exclusively into various sterols, such as cholesterol, via a complex, multi-step pathway. SQS belongs to squalene/phytoene synthase family of proteins. Diversity Squalene synthase has been characterized in animals, plants, and yeast. In terms of structure and mechanics, squalene synthase closely resembles phytoene synthase (PHS), another prenyltransferase. PHS serves a similar role to SQS in plants and bacteria, catalyzing the synthesis of phytoene, a precursor of carotenoid compounds. Structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholesterol
Cholesterol is any of a class of certain organic molecules called lipids. It is a sterol (or modified steroid), a type of lipid. Cholesterol is biosynthesized by all animal cells and is an essential structural component of animal cell membranes. When chemically isolated, it is a yellowish crystalline solid. Cholesterol also serves as a precursor for the biosynthesis of steroid hormones, bile acid and vitamin D. Cholesterol is the principal sterol synthesized by all animals. In vertebrates, hepatic cells typically produce the greatest amounts. It is absent among prokaryotes (bacteria and archaea), although there are some exceptions, such as '' Mycoplasma'', which require cholesterol for growth. François Poulletier de la Salle first identified cholesterol in solid form in gallstones in 1769. However, it was not until 1815 that chemist Michel Eugène Chevreul named the compound "cholesterine". Etymology The word "cholesterol" comes from the Ancient Greek ''chole-'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statin
Statins, also known as HMG-CoA reductase inhibitors, are a class of lipid-lowering medications that reduce illness and mortality in those who are at high risk of cardiovascular disease. They are the most common cholesterol-lowering drugs. Low-density lipoprotein (LDL) carriers of cholesterol play a key role in the development of atherosclerosis and coronary heart disease via the mechanisms described by the lipid hypothesis. Statins are effective in lowering LDL cholesterol and so are widely used for primary prevention in people at high risk of cardiovascular disease, as well as in secondary prevention for those who have developed cardiovascular disease. Side effects of statins include muscle pain, increased risk of diabetes mellitus, and abnormal blood levels of liver enzymes. Additionally, they have rare but severe adverse effects, particularly muscle damage. They inhibit the enzyme HMG-CoA reductase which plays a central role in the production of cholesterol. High cholester ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HMG-CoA Reductase
HMG-CoA reductase (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, official symbol HMGCR) is the rate-controlling enzyme (NADH-dependent, ; NADPH-dependent, ) of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. HMGCR catalyzes the conversion of HMG-CoA to mevalonic acid, a necessary step in the biosynthesis of cholesterol. Normally in mammalian cells this enzyme is competitively suppressed so that its effect is controlled. This enzyme is the target of the widely available cholesterol-lowering drugs known collectively as the statins, which help treat dyslipidemia. HMG-CoA reductase is anchored in the membrane of the endoplasmic reticulum, and was long regarded as having seven transmembrane domains, with the active site located in a long carboxyl terminal domain in the cytosol. More recent evidence shows it to contain eight transmembrane domains. In humans, the gene for HMG-CoA reductase (NADPH) is located on the long arm of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
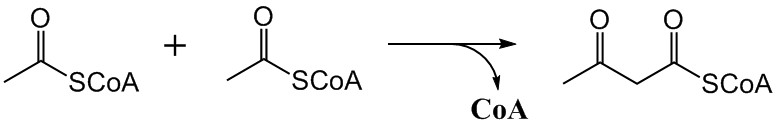
Mevalonate Pathway
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones. The mevalonate pathway begins with acetyl-CoA and ends with the production of IPP and DMAPP. It is best known as the target of statins, a class of cholesterol lowering drugs. Statins inhibit HMG-CoA reductase within the mevalonate pathway. Upper mevalonate pathway The mevalonate pathway of eukaryotes, archaea, and eubacteria all begin the same way. The sole carbon feed stock of the pathway is acetyl-CoA. The first step condenses two acetyl-CoA molecules to yield acetoacetyl-CoA. This is followed by a second condensation to for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prenylation
Prenylation (also known as isoprenylation or lipidation) is the addition of hydrophobic molecules to a protein or a biomolecule. It is usually assumed that prenyl groups (3-methylbut-2-en-1-yl) facilitate attachment to cell membranes, similar to lipid anchors like the GPI anchor, though direct evidence of this has not been observed. Prenyl groups (also called isoprenyl groups, having one hydrogen atom more than isoprene) have been shown to be important for protein–protein binding through specialized prenyl-binding domains. Protein prenylation Protein prenylation involves the transfer of either a farnesyl or a geranylgeranyl moiety to C-terminal cysteine(s) of the target protein. There are three enzymes that carry out prenylation in the cell, farnesyl transferase, Caax protease and geranylgeranyl transferase I. Farnesylation is a type of prenylation, a post-translational modification of proteins by which an isoprenyl group is added to a cysteine residue. It is an important pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liver Damage
Liver disease, or hepatic disease, is any of many diseases of the liver. If long-lasting it is termed chronic liver disease. Although the diseases differ in detail, liver diseases often have features in common. Signs and symptoms Some of the signs and symptoms of a liver disease are the following: * Jaundice * Confusion and altered consciousness caused by hepatic encephalopathy. * Thrombocytopenia and coagulopathy. * Risk of bleeding symptoms particularly taking place in gastrointestinal tract Liver diseases File:Ground glass hepatocytes high mag cropped 2.jpg, Ground glass hepatocytes File:Primary biliary cirrhosis intermed mag much cropping.jpg, Primary biliary cirrhosis File:Buddchiari2.PNG, Budd-chiari syndrome File:Non-alcoholic_fatty_liver_disease1.jpg, Micrograph of non-alcoholic fatty liver disease There are more than a hundred different liver diseases. Some of the most common are: * Fascioliasis, a parasitic infection of liver caused by a liver fluke of the genus ''F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Knockout Mouse
A knockout mouse, or knock-out mouse, is a genetically modified mouse (''Mus musculus'') in which researchers have inactivated, or "knocked out", an existing gene by replacing it or disrupting it with an artificial piece of DNA. They are important animal models for studying the role of genes which have been sequenced but whose functions have not been determined. By causing a specific gene to be inactive in the mouse, and observing any differences from normal behaviour or physiology, researchers can infer its probable function. Mice are currently the laboratory animal species most closely related to humans for which the knockout technique can easily be applied. They are widely used in knockout experiments, especially those investigating genetic questions that relate to human physiology. Gene knockout in rats is much harder and has only been possible since 2003. The first recorded knockout mouse was created by Mario R. Capecchi, Martin Evans, and Oliver Smithies in 1989, for whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypolipidemic Agents
Lipid-lowering agents, also sometimes referred to as hypolipidemic agents, cholesterol-lowering drugs, or antihyperlipidemic agents are a diverse group of pharmaceuticals that are used to lower the level of lipids and lipoproteins such as cholesterol, in the blood (hyperlipidemia). The American Heart Association recommends the descriptor 'lipid lowering agent' be used for this class of drugs rather than the term 'hypolipidemic'. Classes The several classes of lipid lowering drugs may differ in both their impact on the cholesterol profile and adverse effects. For example, some may lower low density lipoprotein (LDL) levels more so than others, while others may preferentially increase high density lipoprotein (HDL). Clinically, the choice of an agent depends on the patient's cholesterol profilecardiovascular risk and the liver and kidney functions of the patient, evaluated against the balancing of risks and benefits of the medications. In the United States, this is guided by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperidines
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, and typical of amines. The name comes from the genus name ''Piper'', which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins. Production Piperidine was first reported in 1850 by the Scottish chemist Thomas Anderson and again, independently, in 1852 by the French chemist Auguste Cahours, who named it. Both of them obtained piperidine by reacting piperine with nitric acid. Industrially, piperidine is produced by the hydrogenation of pyridine, usually over a molybdenum disulfide catalyst: : C5H5N + 3 H2 → C5H10NH Pyridine can also be reduce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
._Key_residues_in_the_central_channel_are_shown_as_spheres..png)