|
Kovar
Kovar (trademark of CRS Holdings, inc., Delaware) is a nickel–cobalt ferrous alloy compositionally identical to Fernico 1, designed to have substantially the same thermal expansion characteristics as borosilicate glass (~5 × 10−6 /K between 30 and 200 °C, to ~10 × 10−6 /K at 800 °C) to allow a tight mechanical joint between the two materials over a range of temperatures. It finds application in glass-to-metal seals in scientific apparatus, and conductors entering glass envelopes of electronic parts such as vacuum tubes (valves), X-ray and microwave tubes and some lightbulbs. Kovar was invented to meet the need for a reliable glass-to-metal seal, which is required in electronic devices such as light bulbs, vacuum tubes, cathode ray tubes, and in vacuum systems in chemistry and other scientific research. Most metals cannot seal to glass because their coefficient of thermal expansion is not the same as glass; as the joint cools after fabrication the stresses d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coefficient Of Thermal Expansion
Thermal expansion is the tendency of matter to change its shape A shape or figure is a graphics, graphical representation of an object or its external boundary, outline, or external Surface (mathematics), surface, as opposed to other properties such as color, Surface texture, texture, or material type. A pl ..., area, volume, and density in response to a change in temperature, usually not including phase transitions. Temperature is a monotonic function of the average molecular kinetic energy of a substance. When a substance is heated, molecules begin to vibrate and move more, usually creating more distance between themselves. Substances which contract with increasing temperature are unusual, and only occur within limited temperature ranges (see examples below). The relative expansion (also called strain (mechanics), strain) divided by the change in temperature is called the material's coefficient of linear thermal expansion and generally varies with temperature. As energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Expansion
Thermal expansion is the tendency of matter to change its shape, area, volume, and density in response to a change in temperature, usually not including phase transitions. Temperature is a monotonic function of the average molecular kinetic energy of a substance. When a substance is heated, molecules begin to vibrate and move more, usually creating more distance between themselves. Substances which contract with increasing temperature are unusual, and only occur within limited temperature ranges (see examples below). The relative expansion (also called strain) divided by the change in temperature is called the material's coefficient of linear thermal expansion and generally varies with temperature. As energy in particles increases, they start moving faster and faster weakening the intermolecular forces between them, therefore expanding the substance. Overview Predicting expansion If an equation of state is available, it can be used to predict the values of the thermal expan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
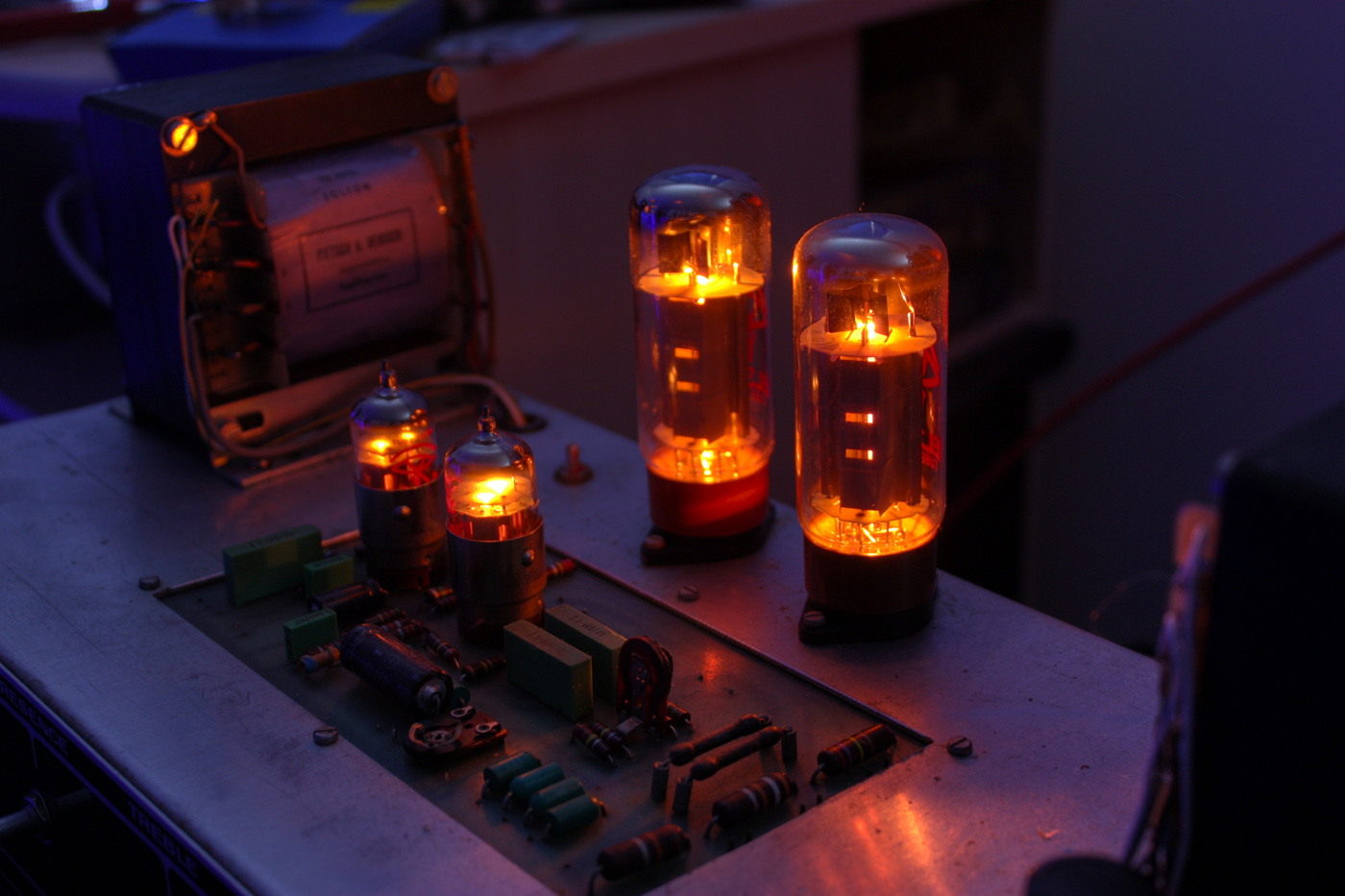
Vacuum Tube
A vacuum tube, electron tube, valve (British usage), or tube (North America), is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied. The type known as a thermionic tube or thermionic valve utilizes thermionic emission of electrons from a hot cathode for fundamental electronic functions such as signal amplifier, amplification and current rectifier, rectification. Non-thermionic types such as a vacuum phototube, however, achieve electron emission through the photoelectric effect, and are used for such purposes as the detection of light intensities. In both types, the electrons are accelerated from the cathode to the anode by the electric field in the tube. The simplest vacuum tube, the diode (i.e. Fleming valve), invented in 1904 by John Ambrose Fleming, contains only a heated electron-emitting cathode and an anode. Electrons can only flow in one direction through the device—fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Tubes
A vacuum tube, electron tube, valve (British usage), or tube (North America), is a device that controls electric current flow in a high vacuum between electrodes to which an electric potential difference has been applied. The type known as a thermionic tube or thermionic valve utilizes thermionic emission of electrons from a hot cathode for fundamental electronic functions such as signal amplification and current rectification. Non-thermionic types such as a vacuum phototube, however, achieve electron emission through the photoelectric effect, and are used for such purposes as the detection of light intensities. In both types, the electrons are accelerated from the cathode to the anode by the electric field in the tube. The simplest vacuum tube, the diode (i.e. Fleming valve), invented in 1904 by John Ambrose Fleming, contains only a heated electron-emitting cathode and an anode. Electrons can only flow in one direction through the device—from the cathode to the anode. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Youngs Modulus
Young's modulus E, the Young modulus, or the modulus of elasticity in tension or compression (i.e., negative tension), is a mechanical property that measures the tensile or compressive stiffness of a solid material when the force is applied lengthwise. It quantifies the relationship between tensile/compressive stress \sigma (force per unit area) and axial strain \varepsilon (proportional deformation) in the linear elastic region of a material and is determined using the formula: E = \frac Young's moduli are typically so large that they are expressed not in pascals but in gigapascals (GPa). Example: * Silly Putty (increasing pressure: length increases quickly, meaning tiny E) * Aluminum (increasing pressure: length increases slowly, meaning high E) Higher Young's modulus corresponds to greater (lengthwise) stiffness. Although Young's modulus is named after the 19th-century British scientist Thomas Young, the concept was developed in 1727 by Leonhard Euler. The first experime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to localized plastic deformation induced by either mechanical indentation or abrasion. In general, different materials differ in their hardness; for example hard metals such as titanium and beryllium are harder than soft metals such as sodium and metallic tin, or wood and common plastics. Macroscopic hardness is generally characterized by strong intermolecular bonds, but the behavior of solid materials under force is complex; therefore, hardness can be measured in different ways, such as scratch hardness, indentation hardness, and rebound hardness. Hardness is dependent on ductility, elastic stiffness, plasticity, strain, strength, toughness, viscoelasticity, and viscosity. Common examples of hard matter are ceramics, concrete, certain metals, and superhard materials, which can be contrasted with soft matter. Measuring hardness There are three main types of hardness measurements: ''scratch' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reduction Of Area
Reduction, reduced, or reduce may refer to: Science and technology Chemistry * Reduction (chemistry), part of a reduction-oxidation (redox) reaction in which atoms have their oxidation state changed. ** Organic redox reaction, a redox reaction that takes place with organic compounds ** Ore reduction: see smelting Computing and algorithms * Reduction (complexity), a transformation of one problem into another problem * Reduction (recursion theory), given sets A and B of natural numbers, is it possible to effectively convert a method for deciding membership in B into a method for deciding membership in A? * Bit Rate Reduction, an audio compression method * Data reduction, simplifying data in order to facilitate analysis * Graph reduction, an efficient version of non-strict evaluation * L-reduction, a transformation of optimization problems which keeps the approximability features * Partial order reduction, a technique for reducing the size of the state-space to be searched by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematically, density is defined as mass divided by volume: : \rho = \frac where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate – this quantity is more specifically called specific weight. For a pure substance the density has the same numerical value as its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium and iridium are the densest known elements at standard conditions for temperature and pressure. To simplify comparisons of density across different s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hot Isostatic Pressing
Hot isostatic pressing (HIP) is a manufacturing process, used to reduce the porosity of metals and increase the density of many ceramic materials. This improves the material's mechanical properties and workability. The process can be used to produce waste form classes. Calcined radioactive waste (waste with additives) is packed into a thin walled metal canister. The adsorbed gases are removed with high heat and the remaining material compressed to full density using argon gas during the heat cycle. This process can shrink steel canisters to minimize space in disposal containers and during transport. It was invented in the 1950s at the Battelle Memorial Institute and has been used to prepare nuclear fuel for submarines since the 1960s. It is used to prepare inactive ceramics as well, and the Idaho National Laboratory has validated it for the consolidation of radioactive ceramic waste forms. ANSTO (Australian Nuclear Science and Technology Organisation) is using HIP as part of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sintering
Clinker nodules produced by sintering Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, plastics, and other materials. The atoms in the materials diffuse across the boundaries of the particles, fusing the particles together and creating one solid piece. Because the sintering temperature does not have to reach the melting point of the material, sintering is often chosen as the shaping process for materials with extremely high melting points such as tungsten and molybdenum. The study of sintering in metallurgical powder-related processes is known as powder metallurgy. An example of sintering can be observed when ice cubes in a glass of water adhere to each other, which is driven by the temperature difference between the water and the ice. Examples of pressure-driven sintering are the compact ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy uses, particularly in stainless steels. It improves strength, workability, and resistance to wear. Manganese oxide is used as an oxidising agent; as a rubber additive; and in glass making, fertilisers, and ceramics. Manganese sulfate can be used as a fungicide. Manganese is also an essential human dietary element, important in macronutrient metabolism, bone formation, and free radical defense systems. It is a critical component in dozens of proteins and enzymes. It is found mostly in the bones, but also the liver, kidneys, and brain. In the human brain, the manganese is bound to manganese metalloproteins, most notably glutamine synthetase in astrocytes. Manganese was first isolated in 1774. It is familiar in the laboratory in the form of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |