|
Ketone Halogenation
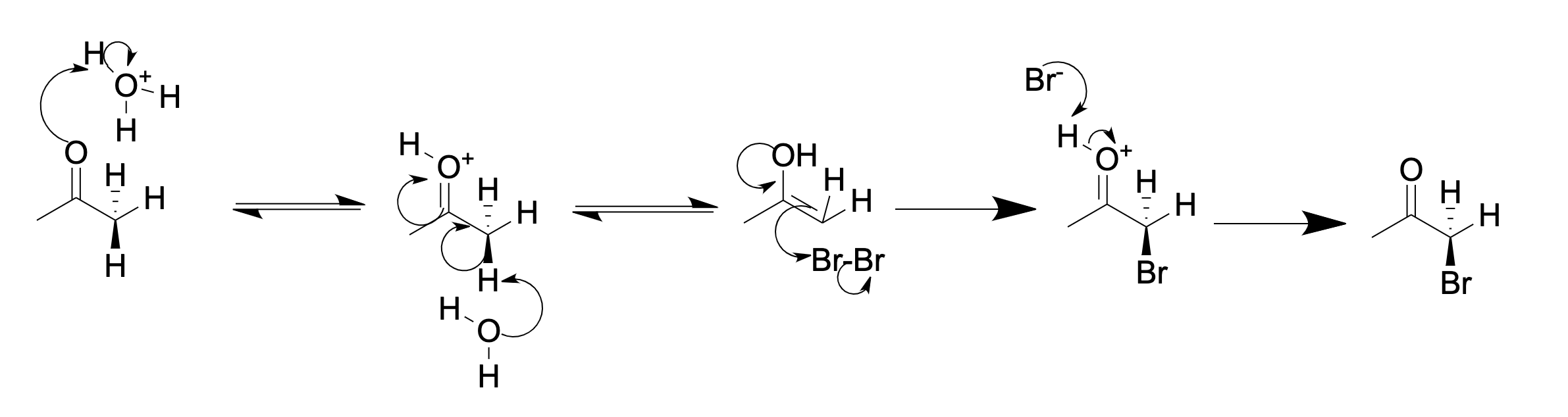
In organic chemistry, α-keto halogenation is a special type of halogenation. The reaction may be carried out under either acidic or basic conditions in an aqueous medium with the corresponding elemental halogen. In this way, chloride, bromide, and iodide (but notably not fluoride) functionality can be inserted selectively in the alpha position of a ketone. The position alpha to the carbonyl group in a ketone is easily halogenated. This is due to its ability to form an enolate in basic solution, or an enol in acidic solution. An example of alpha halogenation is the mono-bromination of acetone, carried out under either acidic or basic conditions, to give bromoacetone: Acidic (in acetic acid): Basic (in aqueous NaOH): In acidic solution, usually only one alpha hydrogen is replaced by a halogen, as each successive halogenation is slower than the first. The halogen decreases the basicity of the carbonyl oxygen, thus making protonation less favorable. However, in basic solut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Mechanism
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequence of database operations that satisfies the ACID properties (which can be perceived as a single logical operation on the data) is called a ''transaction''. For example, a transfer of funds from one bank account to another, even involving multiple changes such as debiting one account and crediting another, is a single transaction. In 1983, Andreas Reuter and Theo Härder coined the acronym ''ACID'', building on earlier work by Jim Gray who named atomicity, consistency, and durability, but not isolation, when characterizing the transaction concept. These four properties are the major guarantees of the transaction paradigm, which has influenced many aspects of development in database systems. According to Gray and Reuter, the IBM Inform ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Green Chemistry
Green chemistry, also called sustainable chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. While environmental chemistry focuses on the effects of polluting chemicals on nature, green chemistry focuses on the environmental impact of chemistry, including lowering consumption of nonrenewable resources and technological approaches for preventing pollution. The overarching goals of green chemistry—namely, more resource-efficient and inherently safer design of molecules, materials, products, and processes—can be pursued in a wide range of contexts. History Green chemistry emerged from a variety of existing ideas and research efforts (such as atom economy and catalysis) in the period leading up to the 1990s, in the context of increasing attention to problems of chemical pollution and resource depletion. The development of green chemistry in Europe a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. History The terms ''nucleophile'' and ''electrophile'' were introduced by Christopher Kelk Ingold in 1933, replacing the terms ''anionoid'' and ''cationoid' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as a placeholder for the aryl group in chemical structure diagrams, analogous to “R” used for any organic substituent. “Ar” is not to be confused with the elemental symbol for argon. A simple aryl group is phenyl (), a group derived from benzene. Examples of other aryl groups consist of: * The tolyl group () which is derived from toluene (methylbenzene) * The xylyl group (), which is derived from xylene (dimethylbenzene) * The naphthyl group (), which is derived from naphthalene Arylation is the process in which an aryl group is attached to a substituent. It is typically achieved by cross-coupling reactions. Nomenclature The most basic aryl group is phenyl, which is made up of a benzene ring with one hydrogen atom substituted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Oxide
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds. Production It is produced on a large scale by pyrometallurgy, as one stage in extracting copper from its ores. The ores are treated with an aqueous mixture of ammonium carbonate, ammonia, and oxygen to give copper(I) and copper(II) ammine complexes, which are extracted from the solids. These complexes are decomposed with steam to give CuO. It can be formed by heating copper in air at around 300–800°C: : 2 Cu + O2 → 2 CuO For laboratory uses, pure copper(II) oxide is better prepared by heating copper(II) nitrate, copper(II) hydroxide, or basic copper(II) carbonate: : 2 Cu(NO3)2(s) → 2 CuO(s) + 4 NO2(g) + O2(g) (180° ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone Halogenation
In organic chemistry, α-keto halogenation is a special type of halogenation. The reaction may be carried out under either acidic or basic conditions in an aqueous medium with the corresponding elemental halogen. In this way, chloride, bromide, and iodide (but notably not fluoride) functionality can be inserted selectively in the alpha position of a ketone. The position alpha to the carbonyl group in a ketone is easily halogenated. This is due to its ability to form an enolate in basic solution, or an enol in acidic solution. An example of alpha halogenation is the mono-bromination of acetone, carried out under either acidic or basic conditions, to give bromoacetone: Acidic (in acetic acid): Basic (in aqueous NaOH): In acidic solution, usually only one alpha hydrogen is replaced by a halogen, as each successive halogenation is slower than the first. The halogen decreases the basicity of the carbonyl oxygen, thus making protonation less favorable. However, in basic solut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Regioselectivity
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where on a substituted benzene ring a further substituent will be added. A specific example is a halohydrin formation reaction with 2-propenylbenzene: : Because of the preference for the formation of one product over another, the reaction is selective. This reaction is regioselective because it selectively generates one constitutional isomer rather than the other. Various examples of regioselectivity have been formulated as rules for certain classes of compounds under certain conditions, many of which are named. Among the first introduced to chemistry students are Markovnikov's rule for the addition of protic acids to alkenes, and the Fürst-Plattner rule for the addition of nucleophiles to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haloform Reaction
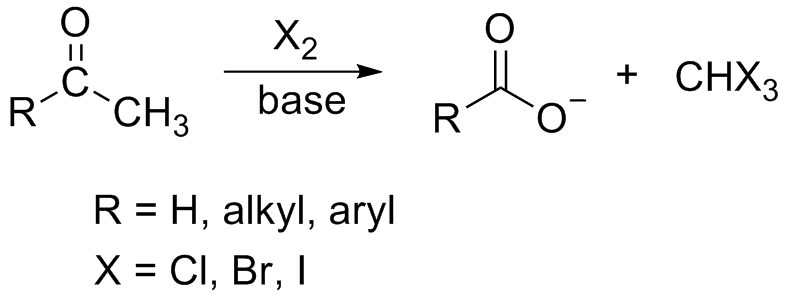
In chemistry, the haloform reaction is a chemical reaction in which a haloform (, where X is a halogen) is produced by the exhaustive halogenation of an acetyl group (, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups () or to produce chloroform (), bromoform (), or iodoform (). Note that fluoroform () can't be prepared in this way. Mechanism In the first step, the halogen dis-proportionates in the presence of hydroxide to give the halide and hypohalite. :Br2 + 2 OH- -> Br- + BrO- + H2O If a secondary alcohol is present, it is oxidized to a ketone by the hypohalite: If a methyl ketone is present, it reacts with the hypohalite in a three-step process: 1. Under basic conditions, the ketone undergoes keto-enol tautomerisation. The enolate undergoes electrophilic attack by the hypohalite (containing a halogen with a formal +1 charge). : 2. When the α(al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoacetone
Bromoacetone is an organic compound with the formula . It is a colorless liquid although impure samples appear yellow or even brown. It is a lachrymatory agent and a precursor to other organic compounds. Occurrence in nature Bromoacetone is present (less than 1%) in the essential oil of a seaweed (''Asparagopsis taxiformis'') from the vicinity of the Hawaiian Islands. Synthesis Bromoacetone is available commercially, sometimes stabilized with magnesium oxide. It was first described in the 19th century, attributed to N. Sokolowsky. Bromoacetone is prepared by combining bromine and acetone, with catalytic acid. As with all ketones, acetone enolizes in the presence of acids or bases. The alpha carbon then undergoes electrophilic substitution with bromine. The main difficulty with this method is over-bromination, resulting in di- and tribrominated products. If a base is present, bromoform is obtained instead, by the haloform reaction. Applications It was used in World War I as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |