|
Isotopes Of Neptunium
Neptunium (93Np) is usually considered an artificial element, although trace quantities are found in nature, so a standard atomic weight cannot be given. Like all trace or artificial elements, it has no stable isotopes. The first isotope to be synthesized and identified was 239Np in 1940, produced by bombarding with neutrons to produce , which then underwent beta decay to . Trace quantities are found in nature from neutron capture reactions by uranium atoms, a fact not discovered until 1951. Twenty-five neptunium radioisotopes have been characterized, with the most stable being with a half-life of 2.14 million years, with a half-life of 154,000 years, and with a half-life of 396.1 days. All of the remaining radioactive isotopes have half-lives that are less than 4.5 days, and the majority of these have half-lives that are less than 50 minutes. This element also has five meta states, with the most stable being (t1/2 22.5 hours). The isotopes of neptunium range from to , t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neptunium
Neptunium is a chemical element with the Symbol (chemistry), symbol Np and atomic number 93. A radioactivity, radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it being named after Neptune, the next planet beyond Uranus. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotrope, allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. It is Radioactive decay, radioactive, radiation poisoning, poisonous, pyrophoricity, pyrophoric, and capable of accumulating in bones, which makes the handling of neptunium dangerous. Although many false claims of its discovery were made over the years, the element was first synthesized by Edwin McMillan and Philip H. Abelson at the Berkeley Radiation Laboratory in 1940. Since then ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Product
In nuclear physics, a decay product (also known as a daughter product, daughter isotope, radio-daughter, or daughter nuclide) is the remaining nuclide left over from radioactive decay. Radioactive decay often proceeds via a sequence of steps (decay chain). For example, 238U decays to 234Th which decays to 234mPa which decays, and so on, to 206Pb (which is stable): : \ce \overbrace^\ce left, upThe decay chain from lead-212 down to lead-208, showing the intermediate decay products In this example: * 234Th, 234mPa,...,206Pb are the decay products of 238U. * 234Th is the daughter of the parent 238U. * 234mPa (234 metastable) is the granddaughter of 238U. These might also be referred to as the daughter products of 238U. (''Depleted Uranium'' — authors: Naomi H. Harley, Ernest C. Foulkes, Lee H. Hil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomeric Transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have half-lives 100 to 1000 times longer than the half-lives of the excited nuclear states that decay with a "prompt" half life (ordinarily on the order of 10−12 seconds). The term "metastable" is usually restricted to isomers with half-lives of 10−9 seconds or longer. Some references recommend 5 × 10−9 seconds to distinguish the metastable half life from the normal "prompt" gamma-emission half-life. Occasionally the half-lives are far longer than this and can last minutes, hours, or years. For example, the nuclear isomer survives so long (at least 1015 years) that it has never been observed to decay spontaneously. The half-life of a nuclear isomer can even exceed that of the ground state of the same nuclide, as shown by as well as , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
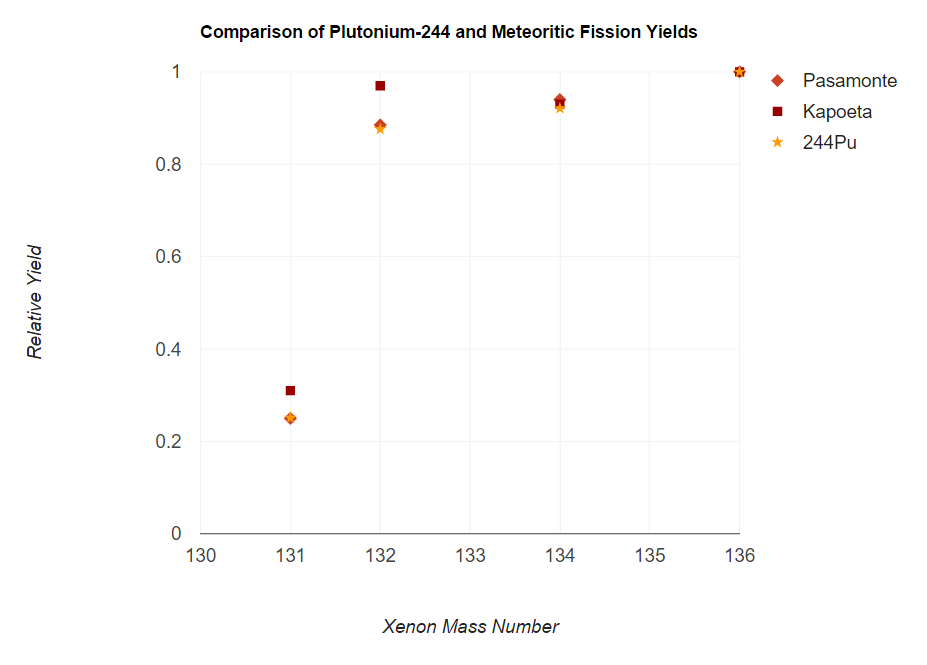
Plutonium-244
Plutonium-244 (244Pu) is an isotope of plutonium that has a half-life of 80 million years. This is longer than any of the other isotopes of plutonium and longer than any other actinide isotope except for the three naturally abundant ones: uranium-235 (704 million years), uranium-238 (4.468 billion years), and thorium-232 (14.05 billion years). Although studies are in conflict, given the mathematics of the decay of plutonium-244, an exceedingly small amount should still be present in the Earth's composition, making plutonium a likely although unproven candidate as the shortest lived primordial element. Natural occurrence Accurate measurements, beginning in the early 1970s, have detected primordial plutonium-244, making it the shortest-lived primordial nuclide. The amount of 244Pu in the pre-Solar nebula (4.57×109 years ago) was estimated as 0.8% the amount of 238U. As the age of the Earth is about 57 half-lives of 244Pu, the amount of plutonium-244 left should be very small; ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cluster Decay
Cluster decay, also named heavy particle radioactivity or heavy ion radioactivity, is a rare type of nuclear decay in which an atomic nucleus emits a small "cluster" of neutrons and protons, more than in an alpha particle, but less than a typical binary fission fragment. Ternary fission into three fragments also produces products in the cluster size. The loss of protons from the parent nucleus changes it to the nucleus of a different element, the daughter, with a mass number ''A''d = ''A'' − ''A''e and atomic number ''Z''d = ''Z'' − ''Z''e, where ''A''e = ''N''e + ''Z''e. For example: : → + This type of rare decay mode was observed in radioisotopes that decay predominantly by alpha emission, and it occurs only in a small percentage of the decays for all such isotopes. The branching ratio with respect to alpha decay is rather small (see the Table below). :B = T_a / T_c Ta and Tc are the half-lives of the parent nucleus relative to alpha decay and cluster radioactivity, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fissile
In nuclear engineering, fissile material is material capable of sustaining a nuclear fission chain reaction. By definition, fissile material can sustain a chain reaction with neutrons of thermal energy. The predominant neutron energy may be typified by either slow neutrons (i.e., a thermal system) or fast neutrons. Fissile material can be used to fuel thermal-neutron reactors, fast-neutron reactors and nuclear explosives. Fissile vs fissionable According to the Ronen fissile rule, for a heavy element with 90 ≤ ''Z'' ≤ 100, its isotopes with , with few exceptions, are fissile (where ''N'' = number of neutrons and ''Z'' = number of protons).The fissile rule thus formulated indicates 33 isotopes as likely fissile: Th-225, 227, 229; Pa-228, 230, 232; U-231, 233, 235; Np-234, 236, 238; Pu-237, 239, 241; Am-240, 242, 244; Cm-243, 245, 247; Bk-246, 248, 250; Cf-249, 251, 253; Es-252, 254, 256; Fm-255, 257, 259. Only fourteen (including a long-lived m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino. : : or when written as a nuclear reaction equation, ^_e + ^_p -> ^_n + ^_ ν_e Since this single emitted neutrino carries the entire decay energy, it has this single characteristic energy. Similarly, the momentum of the neutrino emission causes the daughter atom to recoil with a single characteristic momentum. The resulting daughter nuclide, if it is in an excited state, then transitions to its ground state. Usually, a gamma ray is emitted during this transition, but nuclear de-excitation may also take place by internal conversion. Following capture of an inner electron from the atom, an outer electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spontaneous Fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakdown into smaller nuclei and a few isolated nuclear particles becomes possible at greater atomic mass numbers. History By 1908, physicists understood that alpha decay involved ejection of helium nuclei from a decaying atom. Like cluster decay, alpha decay is not typically categorized as a process of fission. The first nuclear fission process discovered was fission induced by neutrons. Because cosmic rays produce some neutrons, it was difficult to distinguish between induced and spontaneous events. Cosmic rays can be reliably shielded by a thick layer of rock or water. Spontaneous fission was identified in 1940 by Soviet physicists Georgy Flyorov and Konstantin Petrzhak by their observations of uranium in the Moscow Metro Dinamo station ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Review Letters
''Physical Review Letters'' (''PRL''), established in 1958, is a peer-reviewed, scientific journal that is published 52 times per year by the American Physical Society. As also confirmed by various measurement standards, which include the ''Journal Citation Reports'' impact factor and the journal ''h''-index proposed by Google Scholar, many physicists and other scientists consider ''Physical Review Letters'' to be one of the most prestigious journals in the field of physics. ''According to Google Scholar, PRL is the journal with the 9th journal h-index among all scientific journals'' ''PRL'' is published as a print journal, and is in electronic format, online and CD-ROM. Its focus is rapid dissemination of significant, or notable, results of fundamental research on all topics related to all fields of physics. This is accomplished by rapid publication of short reports, called "Letters". Papers are published and available electronically one article at a time. When published in s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an atomic number that is reduced by two. An alpha particle is identical to the nucleus of a helium-4 atom, which consists of two protons and two neutrons. It has a charge of and a mass of . For example, uranium-238 decays to form thorium-234. While alpha particles have a charge , this is not usually shown because a nuclear equation describes a nuclear reaction without considering the electrons – a convention that does not imply that the nuclei necessarily occur in neutral atoms. Alpha decay typically occurs in the heaviest nuclides. Theoretically, it can occur only in nuclei somewhat heavier than nickel (element 28), where the overall binding energy per nucleon is no longer a maximum and the nuclides are therefore unstable toward spontaneou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fallout
Nuclear fallout is the residual radioactive material propelled into the upper atmosphere following a nuclear blast, so called because it "falls out" of the sky after the explosion and the shock wave has passed. It commonly refers to the radioactive dust and ash created when a nuclear weapon explodes. The amount and spread of fallout is a product of the size of the weapon and the altitude at which it is detonated. Fallout may get entrained with the products of a pyrocumulus cloud and fall as black rain (rain darkened by soot and other particulates, which fell within 30–40 minutes of the atomic bombings of Hiroshima and Nagasaki). This radioactive dust, usually consisting of fission products mixed with bystanding atoms that are neutron-activated by exposure, is a form of radioactive contamination. Types of fallout Fallout comes in two varieties. The first is a small amount of carcinogenic material with a long half-life. The second, depending on the height of detonation, is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium-237
Uranium (92U) is a naturally occurring radioactive element that has no stable isotope. It has two primordial isotopes, uranium-238 and uranium-235, that have long half-lives and are found in appreciable quantity in the Earth's crust. The decay product uranium-234 is also found. Other isotopes such as uranium-233 have been produced in breeder reactors. In addition to isotopes found in nature or nuclear reactors, many isotopes with far shorter half-lives have been produced, ranging from 214U to 242U (with the exceptions of 220U and 241U). The standard atomic weight of natural uranium is . Naturally occurring uranium is composed of three major isotopes, uranium-238 (99.2739–99.2752% natural abundance), uranium-235 (0.7198–0.7202%), and uranium-234 (0.0050–0.0059%). All three isotopes are radioactive (i.e., they are radioisotopes), and the most abundant and stable is uranium-238, with a half-life of (close to the age of the Earth). Uranium-238 is an alpha emitter, decaying thr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)


_Halflife_of_Radionulides_depending_on_Z²_to_A_ratio.png)