|
Isotopes Of Indium
Indium (49In) consists of two primordial nuclides, with the most common (~ 95.7%) nuclide (115In) being measurably though weakly radioactive. Its spin-forbidden decay has a half life of 4.41×1014 years. The stable isotope 113In is only 4.3% of naturally occurring indium. Among elements with a known stable isotope, only tellurium and rhenium similarly occur with a stable isotope in lower abundance than the long-lived radioactive isotope. Other than 115In, the longest-lived radioisotope is 111In, with a half-life of 2.8047 days. All other radioisotopes have half-lives less than a day. This element also has 47 isomers, the longest-lived being 114m1In, with a half-life of 49.51 days. All other meta-states have half-lives less than a day, most less than an hour, and many measured in milliseconds or less. Indium-111 is used medically in nuclear imaging, as a radiotracer nuclide tag for gamma camera localization of protein radiopharmaceuticals, such as In-111-labeled octreotide, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts per million of the Earth's crust. Indium has a melting point higher than sodium and gallium, but lower than lithium and tin. Chemically, indium is similar to gallium and thallium, and it is largely intermediate between the two in terms of its properties. Indium was discovered in 1863 by Ferdinand Reich and Hieronymous Theodor Richter by spectroscopic methods. They named it for the indigo blue line in its spectrum. Indium was isolated the next year. Indium is a minor component in zinc sulfide ores and is produced as a byproduct of zinc refinement. It is most notably used in the semiconductor industry, in low-melting-point metal alloys such as solders, in soft-metal high-vacuum seals, and in the production of transparent conductive coati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
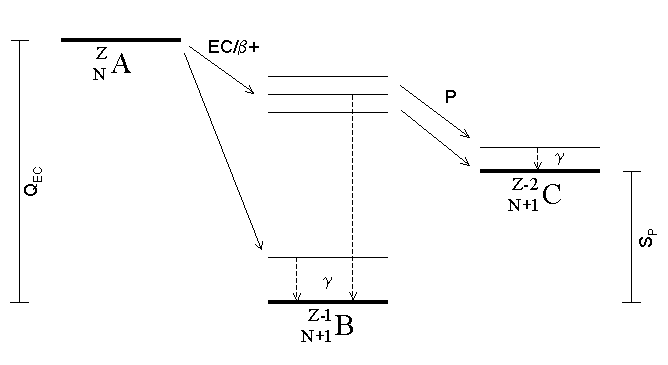
Proton Emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state (or a low-lying isomer) of very proton-rich nuclei, in which case the process is very similar to alpha decay. For a proton to escape a nucleus, the proton separation energy must be negative—the proton is therefore unbound, and tunnels out of the nucleus in a finite time. Proton emission is not seen in naturally occurring isotopes; proton emitters can be produced via nuclear reactions, usually using linear particle accelerators. Although prompt (i.e. not beta-delayed) proton emission was observed from an isomer in cobalt-53 as early as 1969, no other proton-emitting states were found until 1981, when the proton radioactive ground states of lutetium-15 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photoneutron emission and beta-delayed neutron emission. As only a neutron is lost by this process the number of protons remains unchanged, and an atom does not become an atom of a different element, but a different isotope of the same element. Neutrons are also produced in the spontaneous and induced fission of certain heavy nuclides. Spontaneous neutron emission As a consequence of the Pauli exclusion principle, nuclei with an excess of protons or neutrons have a higher average energy per nucleon. Nuclei with a sufficient excess of neutrons have a greater energy than the combination of a free neutron and a nucleus with one less neutron, and therefore can decay by neutron emission. Nuclei which can decay by this process are described as lying beyond the neutron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brookhaven National Laboratory
Brookhaven National Laboratory (BNL) is a United States Department of Energy national laboratory located in Upton, Long Island, and was formally established in 1947 at the site of Camp Upton, a former U.S. Army base and Japanese internment camp. Its name stems from its location within the Town of Brookhaven, approximately 60 miles east of New York City. It is managed by Stony Brook University and Battelle Memorial Institute. Research at BNL includes nuclear and high energy physics, energy science and technology, environmental and bioscience, nanoscience, and national security. The 5,300 acre campus contains several large research facilities, including the Relativistic Heavy Ion Collider and National Synchrotron Light Source II. Seven Nobel Prizes have been awarded for work conducted at Brookhaven Lab. Overview BNL is staffed by approximately 2,750 scientists, engineers, technicians, and support personnel, and hosts 4,000 guest investigators every year. The laboratory has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Nuclear Data Center
The National Nuclear Data Center is an organization based in the Brookhaven National Laboratory that acts as a repository for data regarding nuclear chemistry, such as nuclear structure, decay, and reaction data, as well as historical information regarding previous experiments and literature. According to the ResearchGATE scientific network, "The National Nuclear Data Center NNDC collects, evaluates, and disseminates nuclear physics data for basic nuclear research and applied nuclear technologies." The current Center Head is Dr. Alejandro Sonzogni. History The predecessor group to the NNDC was founded in 1951 when a group known as the Brookhaven Neutron Cross Section Compilation Group was formed at the Brookhaven National Laboratory. In 1955 this group published the reference book "BNL-325," which had to do with the cross-sections of neutrons. After being renamed the Sigma Center, the group was moved to the Reactor Physics Division of the Nuclear Engineering Department in the Brookh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primordial Nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the interstellar medium from which the solar system was formed, and were formed in, or after, the Big Bang, by nucleosynthesis in stars and supernovae followed by mass ejection, by cosmic ray spallation, and potentially from other processes. They are the stable nuclides plus the long-lived fraction of radionuclides surviving in the primordial solar nebula through planet accretion until the present; 286 such nuclides are known. Stability All of the known 251 stable nuclides, plus another 35 nuclides that have half-lives long enough to have survived from the formation of the Earth, occur as primordial nuclides. These 35 primordial radionuclides represent isotopes of 28 separate elements. Cadmium, tellurium, xenon, neodymium, samarium, osmium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spontaneous Fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakdown into smaller nuclei and a few isolated nuclear particles becomes possible at greater atomic mass numbers. History By 1908, physicists understood that alpha decay involved ejection of helium nuclei from a decaying atom. Like cluster decay, alpha decay is not typically categorized as a process of fission. The first nuclear fission process discovered was fission induced by neutrons. Because cosmic rays produce some neutrons, it was difficult to distinguish between induced and spontaneous events. Cosmic rays can be reliably shielded by a thick layer of rock or water. Spontaneous fission was identified in 1940 by Soviet physicists Georgy Flyorov and Konstantin Petrzhak by their observations of uranium in the Moscow Metro Dinamo station ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fission Product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy (kinetic energy of the nuclei), and gamma rays. The two smaller nuclei are the ''fission products''. (See also Fission products (by element)). About 0.2% to 0.4% of fissions are ternary fissions, producing a third light nucleus such as helium-4 (90%) or tritium (7%). The fission products themselves are usually unstable and therefore radioactive. Due to being relatively neutron-rich for their atomic number, many of them quickly undergo beta decay. This releases additional energy in the form of beta particles, antineutrinos, and gamma rays. Thus, fission events normally result in beta and gamma radiation, even though this radiation is not produced directly by the fission event itself. The produced radionuclides have varyi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomeric Transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have half-lives 100 to 1000 times longer than the half-lives of the excited nuclear states that decay with a "prompt" half life (ordinarily on the order of 10−12 seconds). The term "metastable" is usually restricted to isomers with half-lives of 10−9 seconds or longer. Some references recommend 5 × 10−9 seconds to distinguish the metastable half life from the normal "prompt" gamma-emission half-life. Occasionally the half-lives are far longer than this and can last minutes, hours, or years. For example, the nuclear isomer survives so long (at least 1015 years) that it has never been observed to decay spontaneously. The half-life of a nuclear isomer can even exceed that of the ground state of the same nuclide, as shown by as well as , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino. : : or when written as a nuclear reaction equation, ^_e + ^_p -> ^_n + ^_ ν_e Since this single emitted neutrino carries the entire decay energy, it has this single characteristic energy. Similarly, the momentum of the neutrino emission causes the daughter atom to recoil with a single characteristic momentum. The resulting daughter nuclide, if it is in an excited state, then transitions to its ground state. Usually, a gamma ray is emitted during this transition, but nuclear de-excitation may also take place by internal conversion. Following capture of an inner electron from the atom, an outer electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Medicine
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is "radiology done inside out" because it records radiation emitting from within the body rather than radiation that is generated by external sources like X-rays. In addition, nuclear medicine scans differ from radiology, as the emphasis is not on imaging anatomy, but on the function. For such reason, it is called a physiological imaging modality. Single photon emission computed tomography (SPECT) and positron emission tomography (PET) scans are the two most common imaging modalities in nuclear medicine. Diagnostic medical imaging Diagnostic In nuclear medicine imaging, radiopharmaceuticals are taken internally, for example, through inhalation, intravenously or orally. Then, external detectors (gamma cameras) capture and form images from the radiation emitted by the radiopharmaceuticals. This process ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



_Halflife_of_Radionulides_depending_on_Z²_to_A_ratio.png)

