|
Isotopes Of Gadolinium
Naturally occurring gadolinium (64Gd) is composed of 6 stable isotopes, 154Gd, 155Gd, 156Gd, 157Gd, 158Gd and 160Gd, and 1 radioisotope, 152Gd, with 158Gd being the most abundant (24.84% natural abundance). The predicted double beta decay of 160Gd has never been observed; only a lower limit on its half-life of more than 1.3×1021 years has been set experimentally. Thirty-three radioisotopes have been characterized, with the most stable being alpha-decaying 152Gd (naturally occurring) with a half-life of 1.08×1014 years, and 150Gd with a half-life of 1.79×106 years. All of the remaining radioactive isotopes have half-lives less than 74.7 years. The majority of these have half-lives less than 24.6 seconds. Gadolinium isotopes have 10 metastable isomers, with the most stable being 143mGd (t1/2 = 110 seconds), 145mGd (t1/2 = 85 seconds) and 141mGd (t1/2 = 24.5 seconds). The primary decay mode at atomic weights lower than the most abundant stable isotope, 158Gd, is electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen or moisture slowly to form a black coating. Gadolinium below its Curie point of is ferromagnetic, with an attraction to a magnetic field higher than that of nickel. Above this temperature it is the most paramagnetic element. It is found in nature only in an oxidized form. When separated, it usually has impurities of the other rare-earths because of their similar chemical properties. Gadolinium was discovered in 1880 by Jean Charles de Marignac, who detected its oxide by using spectroscopy. It is named after the mineral gadolinite, one of the minerals in which gadolinium is found, itself named for the Finnish chemist Johan Gadolin. Pure gadolinium was first isolated by the chemist Paul-Émile Lecoq de Boisbaudran around 1886. Gadoliniu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Medicine
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is "radiology done inside out" because it records radiation emitting from within the body rather than radiation that is generated by external sources like X-rays. In addition, nuclear medicine scans differ from radiology, as the emphasis is not on imaging anatomy, but on the function. For such reason, it is called a physiological imaging modality. Single photon emission computed tomography (SPECT) and positron emission tomography (PET) scans are the two most common imaging modalities in nuclear medicine. Diagnostic medical imaging Diagnostic In nuclear medicine imaging, radiopharmaceuticals are taken internally, for example, through inhalation, intravenously or orally. Then, external detectors (gamma cameras) capture and form images from the radiation emitted by the radiopharmaceuticals. This process ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
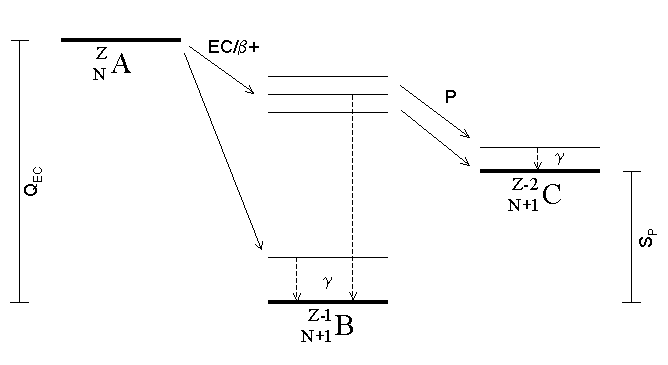
Radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primordial Nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the interstellar medium from which the solar system was formed, and were formed in, or after, the Big Bang, by nucleosynthesis in stars and supernovae followed by mass ejection, by cosmic ray spallation, and potentially from other processes. They are the stable nuclides plus the long-lived fraction of radionuclides surviving in the primordial solar nebula through planet accretion until the present; 286 such nuclides are known. Stability All of the known 251 stable nuclides, plus another 35 nuclides that have half-lives long enough to have survived from the formation of the Earth, occur as primordial nuclides. These 35 primordial radionuclides represent isotopes of 28 separate elements. Cadmium, tellurium, xenon, neodymium, samarium, osmium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino. : : or when written as a nuclear reaction equation, ^_e + ^_p -> ^_n + ^_ ν_e Since this single emitted neutrino carries the entire decay energy, it has this single characteristic energy. Similarly, the momentum of the neutrino emission causes the daughter atom to recoil with a single characteristic momentum. The resulting daughter nuclide, if it is in an excited state, then transitions to its ground state. Usually, a gamma ray is emitted during this transition, but nuclear de-excitation may also take place by internal conversion. Following capture of an inner electron from the atom, an outer electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an atomic number that is reduced by two. An alpha particle is identical to the nucleus of a helium-4 atom, which consists of two protons and two neutrons. It has a charge of and a mass of . For example, uranium-238 decays to form thorium-234. While alpha particles have a charge , this is not usually shown because a nuclear equation describes a nuclear reaction without considering the electrons – a convention that does not imply that the nuclei necessarily occur in neutral atoms. Alpha decay typically occurs in the heaviest nuclides. Theoretically, it can occur only in nuclei somewhat heavier than nickel (element 28), where the overall binding energy per nucleon is no longer a maximum and the nuclides are therefore unstable toward spontaneou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomeric Transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have half-lives 100 to 1000 times longer than the half-lives of the excited nuclear states that decay with a "prompt" half life (ordinarily on the order of 10−12 seconds). The term "metastable" is usually restricted to isomers with half-lives of 10−9 seconds or longer. Some references recommend 5 × 10−9 seconds to distinguish the metastable half life from the normal "prompt" gamma-emission half-life. Occasionally the half-lives are far longer than this and can last minutes, hours, or years. For example, the nuclear isomer survives so long (at least 1015 years) that it has never been observed to decay spontaneously. The half-life of a nuclear isomer can even exceed that of the ground state of the same nuclide, as shown by as well as , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton Emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state (or a low-lying isomer) of very proton-rich nuclei, in which case the process is very similar to alpha decay. For a proton to escape a nucleus, the proton separation energy must be negative—the proton is therefore unbound, and tunnels out of the nucleus in a finite time. Proton emission is not seen in naturally occurring isotopes; proton emitters can be produced via nuclear reactions, usually using linear particle accelerators. Although prompt (i.e. not beta-delayed) proton emission was observed from an isomer in cobalt-53 as early as 1969, no other proton-emitting states were found until 1981, when the proton radioactive ground states of lutetium-15 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioisotope Thermoelectric Generators
A radioisotope thermoelectric generator (RTG, RITEG), sometimes referred to as a radioisotope power system (RPS), is a type of nuclear battery that uses an array of thermocouples to convert the heat released by the decay of a suitable radioactive material into electricity by the Seebeck effect. This type of generator has no moving parts. RTGs have been used as power sources in satellites, space probes, and uncrewed remote facilities such as a series of lighthouses built by the Soviet Union inside the Arctic Circle. RTGs are usually the most desirable power source for unmaintained situations that need a few hundred watts (or less) of power for durations too long for fuel cells, batteries, or generators to provide economically, and in places where solar cells are not practical. Safe use of RTGs requires containment of the radioisotopes long after the productive life of the unit. The expense of RTGs tends to limit their use to niche applications in rare or special situations. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' "lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Separation
Isotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes. The use of the nuclides produced is varied. The largest variety is used in research (e.g. in chemistry where atoms of "marker" nuclide are used to figure out reaction mechanisms). By tonnage, separating natural uranium into enriched uranium and depleted uranium is the largest application. In the following text, mainly the uranium enrichment is considered. This process is crucial in the manufacture of uranium fuel for nuclear power plants, and is also required for the creation of uranium-based nuclear weapons. Plutonium-based weapons use plutonium produced in a nuclear reactor, which must be operated in such a way as to produce plutonium already of suitable isotopic mix or ''grade''. While different chemical elements can be purified through chemical reaction, chemical processes, isotopes of the same element have nearly identical chemical properties, which makes this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |






_(cropped).jpg)