|
Isotopes Of Europium
Naturally occurring europium (63Eu) is composed of two isotopes, 151Eu and 153Eu, with 153Eu being the most abundant (52.2% natural abundance). While 153Eu is observationally stable, 151Eu was found in 2007 to be unstable and undergo alpha decay. The half-life is measured to be (4.62 ± 0.95(stat.) ± 0.68(syst.)) × 1018 years which corresponds to 1 alpha decay per two minutes in every kilogram of natural europium. Besides the natural radioisotope 151Eu, 36 artificial radioisotopes have been characterized, with the most stable being 150Eu with a half-life of 36.9 years, 152Eu with a half-life of 13.516 years, 154Eu with a half-life of 8.593 years, and 155Eu with a half-life of 4.7612 years. The majority of the remaining radioactive isotopes, which range from 130Eu to 170Eu, have half-lives that are less than 12.2 seconds. This element also has 18 meta states, with the most stable being 150mEu (t1/2 12.8 hours), 152m1Eu (t1/2 9.3116 hours) and 152m2Eu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lanthanide, as it can be dented with a fingernail and easily cut with a knife. When oxidation is removed a shiny-white metal is visible. Europium was isolated in 1901 and is named after the continent of Europe. Being a typical member of the lanthanide series, europium usually assumes the oxidation state +3, but the oxidation state +2 is also common. All europium compounds with oxidation state +2 are slightly reducing. Europium has no significant biological role and is relatively non-toxic as compared to other heavy metals. Most applications of europium exploit the phosphorescence of europium compounds. Europium is one of the rarest of the rare-earth elements on Earth.Stwertka, Albert. ''A Guide to the Elements'', Oxford University Press, 1996, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primordial Nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the interstellar medium from which the solar system was formed, and were formed in, or after, the Big Bang, by nucleosynthesis in stars and supernovae followed by mass ejection, by cosmic ray spallation, and potentially from other processes. They are the stable nuclides plus the long-lived fraction of radionuclides surviving in the primordial solar nebula through planet accretion until the present; 286 such nuclides are known. Stability All of the known 251 stable nuclides, plus another 35 nuclides that have half-lives long enough to have survived from the formation of the Earth, occur as primordial nuclides. These 35 primordial radionuclides represent isotopes of 28 separate elements. Cadmium, tellurium, xenon, neodymium, samarium, osmium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
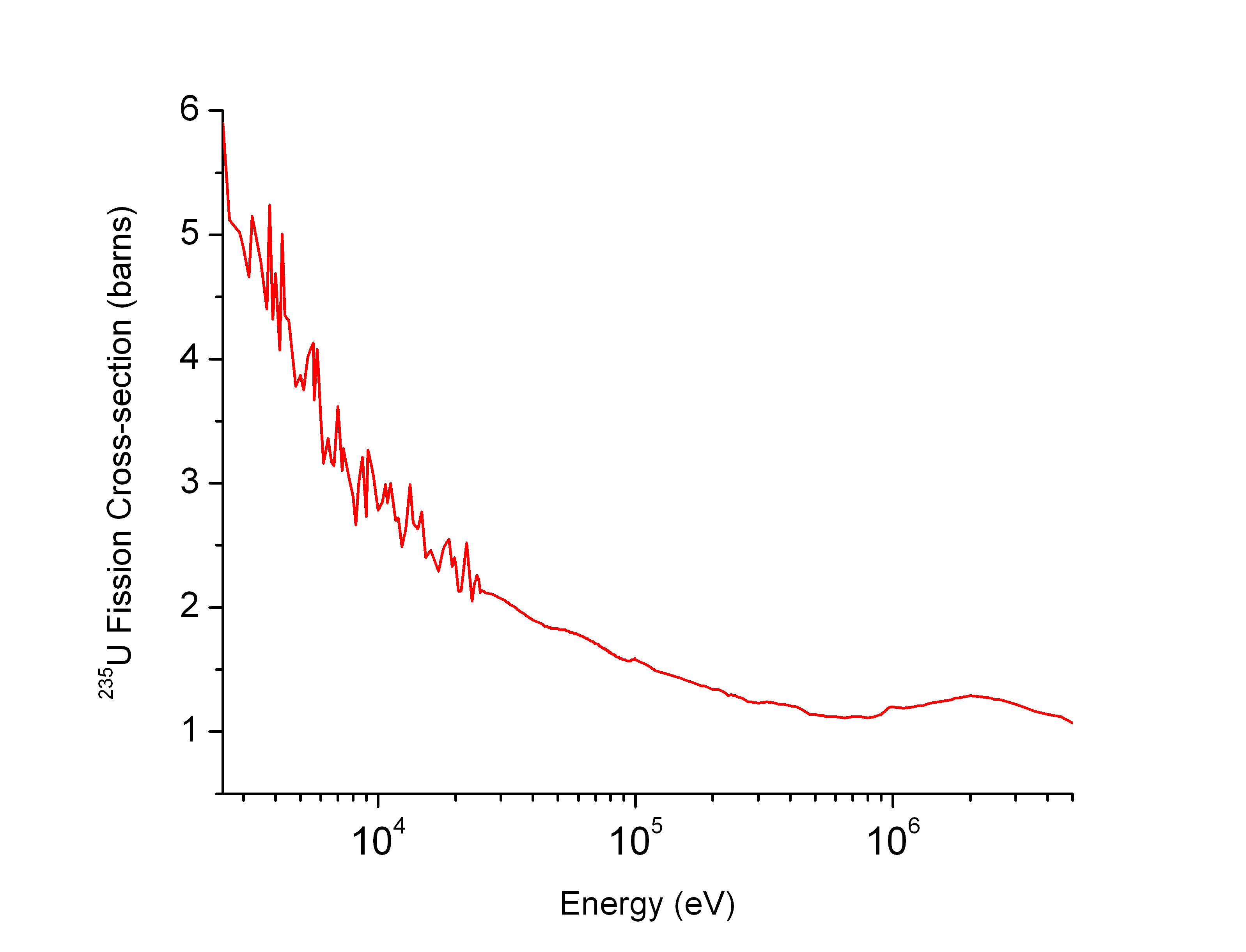
Resonance Integral
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled electrostatically. Neutron capture plays a significant role in the cosmic nucleosynthesis of heavy elements. In stars it can proceed in two ways: as a rapid process (r-process) or a slow process (s-process). Nuclei of masses greater than 56 cannot be formed by thermonuclear reactions (i.e., by nuclear fusion) but can be formed by neutron capture. Neutron capture on protons yields a line at 2.223 MeV predicted and commonly observed in solar flares. Neutron capture at small neutron flux At small neutron flux, as in a nuclear reactor, a single neutron is captured by a nucleus. For example, when natural gold (197Au) is irradiated by neutrons (n), the isotope 198Au is formed in a highly excited state, and quickly deca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Neutrons
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium with a certain temperature. The neutron energy distribution is then adapted to the Maxwell distribution known for thermal motion. Qualitatively, the higher the temperature, the higher the kinetic energy of the free neutrons. The momentum and wavelength of the neutron are related through the de Broglie relation. The large wavelength of slow neutrons allows for the large cross section. Neutron energy distribution ranges But different ranges with different names are observed in other sources. The following is a detailed classification: Thermal A thermal neutron is a free neutron with a kinetic energy of about 0.025 eV (about 4.0×10−21 J or 2.4 MJ/kg, hence a speed of 2.19 km/s), which is the energy corresponding to the most pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Cross-section
In nuclear physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of neutron-nuclei reactions taking place is equal to the product of the number of incident neutrons that would pass through the area and the number of target nuclei. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power of a nuclear power plant. The standard unit for measuring the cross section is the barn, which is equal to 10−28 m2 or 10−24 cm2. The larger the neutron cross section, the more likely a neutron will react with the nucleus. An isotope (or nuclide) can be classified according to its neutron cross section and how it reacts to an incident neutron. Nuclides that tend to absorb a neutron and either decay or keep the neutron in its nucleus are neutron absor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled electrostatically. Neutron capture plays a significant role in the cosmic nucleosynthesis of heavy elements. In stars it can proceed in two ways: as a rapid process (r-process) or a slow process (s-process). Nuclei of masses greater than 56 cannot be formed by thermonuclear reactions (i.e., by nuclear fusion) but can be formed by neutron capture. Neutron capture on protons yields a line at 2.223 MeV predicted and commonly observed in solar flares. Neutron capture at small neutron flux At small neutron flux, as in a nuclear reactor, a single neutron is captured by a nucleus. For example, when natural gold (197Au) is irradiated by neutrons (n), the isotope 198Au is formed in a highly excited state, and quickly deca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fission Product Yield
Nuclear fission splits a heavy nucleus such as uranium or plutonium into two lighter nuclei, which are called fission products. Yield refers to the fraction of a fission product produced per fission. Yield can be broken down by: # Individual isotope # Chemical element spanning several isotopes of different mass number but same atomic number. # Nuclei of a given mass number regardless of atomic number. Known as "chain yield" because it represents a decay chain of beta decay. Isotope and element yields will change as the fission products undergo beta decay, while chain yields do not change after completion of neutron emission by a few neutron-rich initial fission products (delayed neutrons), with half-life measured in seconds. A few isotopes can be produced directly by fission, but not by beta decay because the would-be precursor with atomic number one greater is stable and does not decay. Chain yields do not account for these "shadowed" isotopes; however, they have very low yie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Power Plant
A nuclear power plant (NPP) is a thermal power station in which the heat source is a nuclear reactor. As is typical of thermal power stations, heat is used to generate steam that drives a steam turbine connected to a electric generator, generator that produces electricity. , the International Atomic Energy Agency reported there were 422 nuclear power reactors in operation in 32 countries around the world, and 57 nuclear power reactors under construction. Nuclear plants are very often used for base load since their operations, maintenance, and fuel costs are at the lower end of the spectrum of costs. However, building a nuclear power plant often spans five to ten years, which can accrue to significant financial costs, depending on how the initial investments are financed. Nuclear power plants have a carbon footprint comparable to that of renewable energy such as photovoltaic power station, solar farms and wind farms, and much lower than fossil fuels such as gas-fired ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Reactor
A thermal-neutron reactor is a nuclear reactor that uses slow or thermal neutrons. ("Thermal" does not mean hot in an absolute sense, but means in thermal equilibrium with the medium it is interacting with, the reactor's fuel, moderator and structure, which is much lower energy than the fast neutrons initially produced by fission.) Most nuclear power plant reactors are thermal reactors and use a neutron moderator to slow neutrons until they approach the average kinetic energy of the surrounding particles, that is, to reduce the speed of the neutrons to low-velocity, thermal neutrons. Neutrons are uncharged, this allows them to penetrate deep in the target and close to the nuclei,Squires, G.L. (2012, March 29). Introduction of the Theory of Thermal Neutron Scattering. https://books.google.com/books?hl=en&lr=&id=KUVD8KJt7_0C&oi=fnd&pg=PR9&dq=thermal-neutron+reactor&ots=1tn_4dppSF&sig=QDWkMU5-iW8_4GCXjItypUchKBQ#v=onepage&q=thermal-neutron%20reactor&f=false thus scattering neutrons by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Energy
The decay energy is the energy change of a nucleus having undergone a radioactive decay. Radioactive decay is the process in which an unstable atomic nucleus loses energy by emitting ionizing particles and radiation. This decay, or loss of energy, results in an atom of one type (called the parent nuclide) transforming to an atom of a different type (called the daughter nuclide). Decay calculation The energy difference of the reactants is often written as ''Q'': :Q = \left( \text \right)_\text - \left( \text \right)_\text, :Q = \left(\text \right)_ c^2 - \left( \text \right )_\text c^2 . Decay energy is usually quoted in terms of the energy units MeV (million electronvolts) or keV (thousand electronvolts): : Q \text = -931.5 \Delta M \text,~~(\text\Delta M = \Sigma M_\text - \Sigma M_\text). Types of radioactive decay include * gamma ray * beta decay (decay energy is divided between the emitted electron and the neutrino which is emitted at the same time) * alpha decay The deca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fission Product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy (kinetic energy of the nuclei), and gamma rays. The two smaller nuclei are the ''fission products''. (See also Fission products (by element)). About 0.2% to 0.4% of fissions are ternary fissions, producing a third light nucleus such as helium-4 (90%) or tritium (7%). The fission products themselves are usually unstable and therefore radioactive. Due to being relatively neutron-rich for their atomic number, many of them quickly undergo beta decay. This releases additional energy in the form of beta particles, antineutrinos, and gamma rays. Thus, fission events normally result in beta and gamma radiation, even though this radiation is not produced directly by the fission event itself. The produced radionuclides have varyi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Physical Journal A
The ''European Physical Journal A: Hadrons and Nuclei'' is an academic journal, recognized by the European Physical Society, presenting new and original research results in a variety of formats, including Regular Articles, Reviews, Tools for Experiment and Theory/Scientific Notes and Letters. Topics covered include: ;Hadron Physics: *Structure and Dynamics of Hadrons *Baryon and Meson Spectroscopy *Hadronic and Electroweak Interactions of Hadrons *Nonperturbative Approaches to QCD *Phenomenological Approaches to Hadron Physics ;Nuclear Physics: *Nuclear Structure and Reactions *Structure and function of nanostructures *Few-Body and Many-Body Systems *Heavy-Ion Physics *Hypernuclei *Radioactive Beams *Nuclear Astrophysics History Prior to 1998, the journal was named ''Zeitschrift für Physik A Hadrons and Nuclei''. Thomas Walcher's term as Editor-in-Chief of EPJ A came to an end in 2006. In January 2007 Enzo de Sanctis started as new Editor-in-Chief and he was joined in July that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |