|
Isotope Shift
The isotopic shift (also called isotope shift) is the shift in various forms of spectroscopy that occurs when one nuclear isotope is replaced by another. NMR spectroscopy In NMR spectroscopy, Isotopic effects on chemical shifts are typically small, far less than 1 ppm the typical unit for measuring shifts. The NMR signals for and ("HD") are readily distinguished in terms of their chemical shifts. The asymmetry of the signal for the "protio" impurity in arises from the differing chemical shifts of and . Vibrational spectra Isotopic shifts are best known and most widely used in vibration spectroscopy where the shifts are large, being proportional to the ratio of the square root of the isotopic masses. In the case of hydrogen, the "H-D shift" is (1/2)1/2 or 1/1.41. Thus, the (totally symmetric) C-H vibration for and occur at 2917 cm−1 and 2109 cm−1, respectively. This shift reflects the differing reduced mass for the affected bonds. Atomic spectra I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO) In simpler terms, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum. Historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. Spectroscopy, primarily in the electromagnetic spectrum, is a fundamental exploratory tool in the fields of astronomy, chemistry, materials science, and physics, allowing the composition, physical structure and e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Constant
In spectroscopy, the Rydberg constant, symbol R_\infty for heavy atoms or R_\text for hydrogen, named after the Swedish physicist Johannes Rydberg, is a physical constant relating to the electromagnetic spectra of an atom. The constant first arose as an empirical fitting parameter in the Rydberg formula for the hydrogen spectral series, but Niels Bohr later showed that its value could be calculated from more fundamental constants via his Bohr model. Before the redefinition of the SI base units in , R_\infty and the electron spin ''g''-factor were the most accurately measured physical constants. The constant is expressed for either hydrogen as R_\text, or at the limit of infinite nuclear mass as R_\infty. In either case, the constant is used to express the limiting value of the highest wavenumber (inverse wavelength) of any photon that can be emitted from an atom, or, alternatively, the wavenumber of the lowest-energy photon capable of ionizing an atom from its ground state. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinetic Isotope Effect
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for the reactions involving the light (''kL'') and the heavy (''kH'') isotopically substituted reactants (isotopologues): :\text=\frac This change in reaction rate is a quantum mechanical effect that primarily results from heavier isotopologues having lower vibrational frequencies compared to their lighter counterparts. In most cases, this implies a greater energetic input needed for heavier isotopologues to reach the transition state (or, in rare cases, the dissociation limit), and consequently, a slower reaction rate. The study of kinetic isotope effects can help the elucidation of the reaction mechanism of certain chemical reactions and is occasionally exploited in drug development to improve unfavorable pharmacokinetics by protecting m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perturbation Theory (quantum Mechanics)
In quantum mechanics, perturbation theory is a set of approximation schemes directly related to mathematical perturbation for describing a complicated quantum system in terms of a simpler one. The idea is to start with a simple system for which a mathematical solution is known, and add an additional "perturbing" Hamiltonian representing a weak disturbance to the system. If the disturbance is not too large, the various physical quantities associated with the perturbed system (e.g. its energy levels and eigenstates) can be expressed as "corrections" to those of the simple system. These corrections, being small compared to the size of the quantities themselves, can be calculated using approximate methods such as asymptotic series. The complicated system can therefore be studied based on knowledge of the simpler one. In effect, it is describing a complicated unsolved system using a simple, solvable system. Approximate Hamiltonians Perturbation theory is an important tool for de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Model
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force. The diameter of the nucleus is in the range of () for hydrogen (the diameter of a single proton) to about for uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 26,634 (uranium ato ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Academic Press
Academic Press (AP) is an academic book publisher founded in 1941. It was acquired by Harcourt, Brace & World in 1969. Reed Elsevier bought Harcourt in 2000, and Academic Press is now an imprint of Elsevier. Academic Press publishes reference books, serials and online products in the subject areas of: * Communications engineering * Economics * Environmental science * Finance * Food science and nutrition * Geophysics * Life sciences * Mathematics and statistics * Neuroscience * Physical sciences * Psychology Well-known products include the ''Methods in Enzymology'' series and encyclopedias such as ''The International Encyclopedia of Public Health'' and the ''Encyclopedia of Neuroscience''. See also * Akademische Verlagsgesellschaft (AVG) — the German predecessor, founded in 1906 by Leo Jolowicz (1868–1940), the father of Walter Jolowicz Walter may refer to: People * Walter (name), both a surname and a given name * Little Walter, American blues harmonica player Marion Wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wave Function
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum system. The wave function is a complex-valued probability amplitude, and the probabilities for the possible results of measurements made on the system can be derived from it. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi, respectively). The wave function is a function of the degrees of freedom corresponding to some maximal set of commuting observables. Once such a representation is chosen, the wave function can be derived from the quantum state. For a given system, the choice of which commuting degrees of freedom to use is not unique, and correspondingly the domain of the wave function is also not unique. For instance, it may be taken to be a function of all the position coordinates of the particles over position space, or the momenta of all the particles over momentum space; the two are related by a Fourier tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hantaro Nagaoka
was a Japanese physicist and a pioneer of Japanese physics during the Meiji period. Life Nagaoka was born in Nagasaki, Japan on August 19, 1865 and educated at the University of Tokyo. After graduating with a degree in physics in 1887, Nagaoka worked with a visiting Scottish physicist, Cargill Gilston Knott, on early problems in magnetism, namely magnetostriction in liquid nickel. In 1893, Nagaoka traveled to Europe, where he continued his education at the universities of Berlin, Munich, and Vienna, including courses on Saturn's rings and a course with Ludwig Boltzmann on his Kinetic Theory of Gases, two influences which would be reflected in Nagaoka's later work. Nagaoka also attended, in 1900, the First International Congress of Physicists in Paris, where he heard Marie Curie lecture on radioactivity, an event that aroused Nagaoka's interest in atomic physics. Nagaoka returned to Japan in 1901 and served as professor of physics at Tokyo University until 1925. After his reti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Formula
In atomic physics, the Rydberg formula calculates the wavelengths of a spectral line in many chemical elements. The formula was primarily presented as a generalization of the Balmer series for all atomic electron transitions of hydrogen. It was first empirically stated in 1888 by the Swedish physicist Johannes Rydberg, then theoretically by Niels Bohr in 1913, who used a primitive form of quantum mechanics. The formula directly generalizes the equations used to calculate the wavelengths of the hydrogen spectral series. History In 1880, Rydberg worked on a formula describing the relation between the wavelengths in spectral lines of alkali metals. He noticed that lines came in series and he found that he could simplify his calculations using the wavenumber (the number of waves occupying the unit length, equal to 1/''λ'', the inverse of the wavelength) as his unit of measurement. He plotted the wavenumbers (''n'') of successive lines in each series against consecutive integers w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties. The term isotope is formed from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in 1913 in a suggestion to the British chemist Frederick Soddy. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
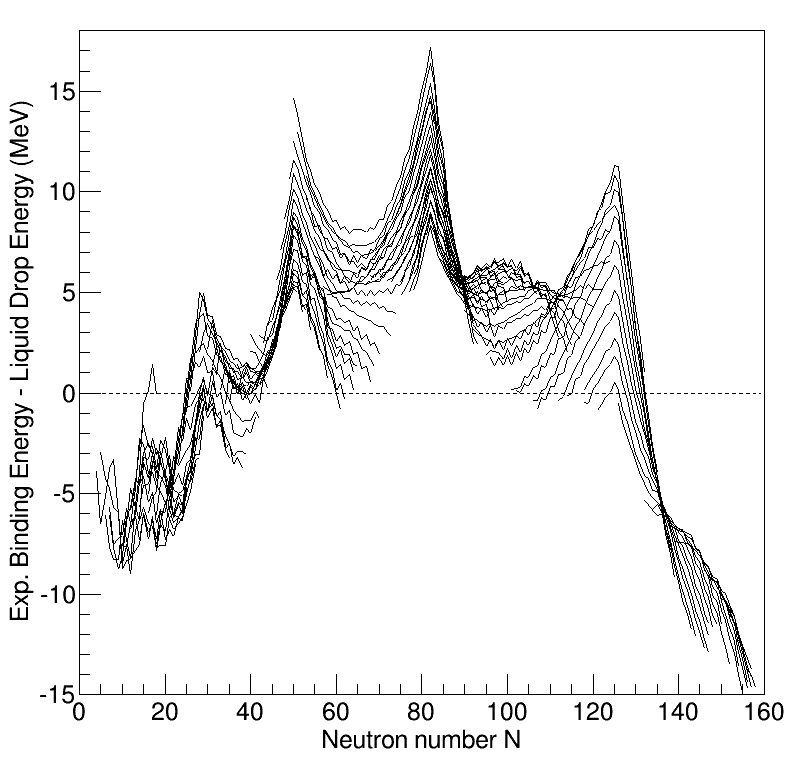
Nuclear Structure
Understanding the structure of the atomic nucleus is one of the central challenges in nuclear physics. Models The liquid drop model The liquid drop model is one of the first models of nuclear structure, proposed by Carl Friedrich von Weizsäcker in 1935. It describes the nucleus as a semiclassical fluid made up of neutrons and protons, with an internal repulsive electrostatic force proportional to the number of protons. The quantum mechanical nature of these particles appears via the Pauli exclusion principle, which states that no two nucleons of the same kind can be at the same state. Thus the fluid is actually what is known as a Fermi liquid. In this model, the binding energy of a nucleus with Z protons and N neutrons is given by :E_ = a_ A - a_ A^ - a_ \frac - a_ \frac - \delta(A,Z) where A=Z+N is the total number of nucleons ( Mass Number). The terms proportional to A and A^ represent the volume and surface energy of the liquid drop, the term proportional to Z^ repre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperfine Structure
In atomic physics, hyperfine structure is defined by small shifts in otherwise degenerate energy levels and the resulting splittings in those energy levels of atoms, molecules, and ions, due to electromagnetic multipole interaction between the nucleus and electron clouds. In atoms, hyperfine structure arises from the energy of the nuclear magnetic dipole moment interacting with the magnetic field generated by the electrons and the energy of the nuclear electric quadrupole moment in the electric field gradient due to the distribution of charge within the atom. Molecular hyperfine structure is generally dominated by these two effects, but also includes the energy associated with the interaction between the magnetic moments associated with different magnetic nuclei in a molecule, as well as between the nuclear magnetic moments and the magnetic field generated by the rotation of the molecule. Hyperfine structure contrasts with '' fine structure'', which results from the interaction b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_(2).png)