|
Isonitriles
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds. Properties Structure and bonding The C-N distance in isocyanides is 115.8 pm in methyl isocyanide. The C-N-C angles are near 180°. Akin to carbon monoxide, isocyanides are described by two resonance structures, one with a triple bond between the nitrogen and the carbon and one with a double bond between. The π lone pair of the nitrogen stabilizes the structure and is responsible of the linearity of isocyanides, although the reactivity of isocyanides reflects some carbene character, at least in a formal sense. Thus, both resonance structures are useful representations. They are sus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Oxychloride
Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula . It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate. Structure Like phosphate, is tetrahedral in shape. It features three P−Cl bonds and one strong P=O double bond, with an estimated bond dissociation energy of 533.5 kJ/mol. On the basis of bond length and electronegativity, the Schomaker-Stevenson rule suggests that the double bond form is dominant, in contrast with the case of . The P=O bond involves the donation of the lone pair electrons on oxygen ''p''-orbitals to the antibonding combinations associated with phosphorus-chlorine bonds, thus constituting ''π'' bonding. Phosphoryl chloride exists as neutral molecules in the solid, liquid and gas s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance (chemistry)
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is sufficient for describing the chemical bonding and rationalizing experimentally determined molecular properties like bond lengths, angles, and dipole moment. Howev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydration Reaction
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction. Dehydration reactions in organic chemistry Esterification The classic example of a dehydration reaction is the Fischer esterification, which involves treating a carboxylic acid with an alcohol to give an ester :RCO2H + R′OH RCO2R′ + H2O Often such reactions require the presence of a dehydrating agent, i.e. a substance that reacts with water. Etherification Two monosaccharides, such as glucose and fructose, can be joined together (to form saccharose) using dehydration synthesis. The new molecule, consisting of two monosaccharides, is called a disaccharide. Nitrile formation Nitriles are often prepared by dehydration of primary amides. :RC(O)NH2 → RCN + H2O Ketene formation Ketene is produced by heating acetic acid and trapping the product: :CH3CO2H → CH2=C= ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some stag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tert-Butylamine
''tert''-Butylamine is an organic chemical compound with the formula (CH3)3CNH2. It is a colorless liquid with a typical amine-like odor. ''tert''-Butylamine is one of the four isomeric amines of butane, the others being ''n''-butylamine, ''sec''-butylamine and isobutylamine. Preparation ''tert''-Butylamine is produced commercially by direct amination of isobutylene using zeolite catalysts: :NH3 + CH2=C(CH3)2 → H2NC(CH3)3 The Ritter reaction of isobutene with hydrogen cyanide is not useful because it produces too much waste. :(CH3)2C=CH2 + HCN + H2O → (CH3)3CNHCHO :(CH3)3CNHCHO + H2O → (CH3)3CNH2 + HCO2H In the laboratory, it can be prepared by the hydrogenolysis of 2,2-dimethylethylenimine, or via ''tert''-butylphthalimide. Uses ''tert''-Butylamine is used as an intermediate in the preparation of the sulfenamides such as ''N''-''tert''-butyl-2-benzothiazylsulfenamide and ''N''-''tert''-butyl-2-benzothiazylsulfenimide. As rubber accelerators, thes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichlorocarbene
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapidly inserts into other bonds. Preparation Dichlorocarbene is most commonly generated by reaction of chloroform and a base such as potassium ''tert''-butoxide or aqueous sodium hydroxide. A phase transfer catalyst, for instance benzyltriethylammonium bromide, facilitates the migration of the hydroxide in the organic phase. :HCCl3 + NaOH → CCl2 + NaCl + H2O Other reagents and routes Another precursor to dichlorocarbene is ethyl trichloroacetate. Upon treatment with sodium methoxide it releases CCl2. Phenyl(trichloromethyl)mercury decomposes thermally to release CCl2. :PhHgCCl3 → CCl2 + PhHgCl Dichlorodiazirine, which is stable in the dark, decomposes into dichlorocarbene and nitrogen via photolysis. Dichlorocarbene can also be obta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroform
Chloroform, or trichloromethane, is an organic compound with chemical formula, formula Carbon, CHydrogen, HChlorine, Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is trihalomethane. It is a powerful anesthetic, euphoriant, anxiolytic, and sedative when inhaled or ingested. Structure The molecule adopts a tetrahedral molecular geometry with C3v symmetry group, symmetry. Natural occurrence The total global flux of chloroform through the environment is approximately tonnes per year, and about 90% of emissions are natural in origin. Many kinds of seaweed produce chloroform, and fungi are believed to produce chloroform in soil. Abiotic processes are also believed to contribute to natural chloroform productions in soils although the mechanism is still unclear. Chloroform volatilizes readily from soil and surface water and undergoes degradation in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
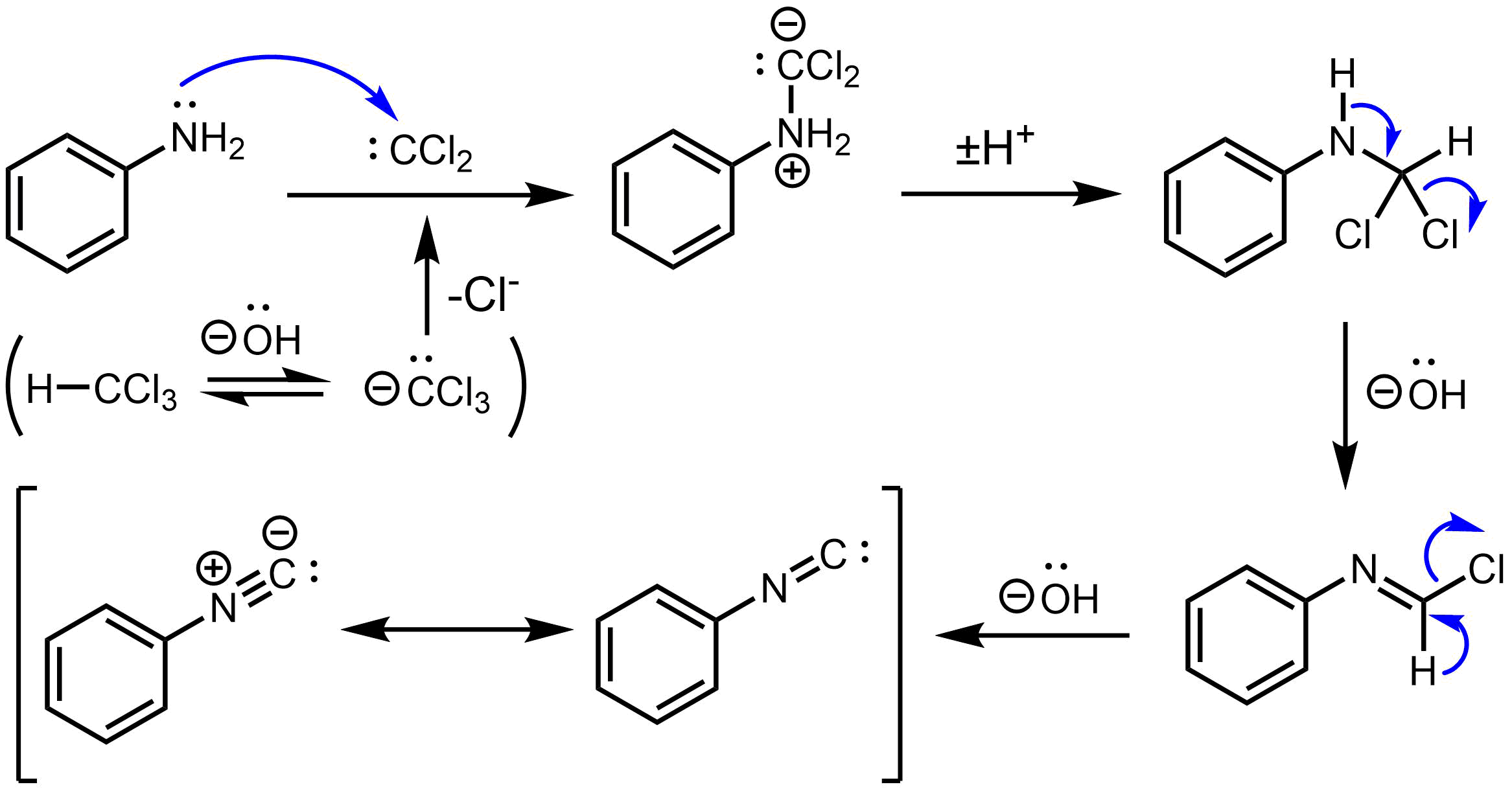
Carbylamine Reaction
The carbylamine reaction (also known as the Hoffmann isocyanide synthesis) is the synthesis of an isocyanide by the reaction of a primary amine, chloroform, and base. The conversion involves the intermediacy of dichlorocarbene. Illustrative is the synthesis of ''tert''-butyl isocyanide from ''tert''-butylamine in the presence of catalytic amount of the phase transfer catalyst benzyltriethylammonium chloride. :Me3CNH2 + CHCl3 + 3 NaOH → Me3CNC + 3 NaCl + 3 H2O Similar reactions have been reported for aniline. It is used to prepare secondary amines. Test for primary amines As it is only effective for primary amines, the carbylamine reaction can be used as a chemical test for their presence. In this context, the reaction is also known as Saytzeff's isocyanide test. In this reaction, the analyte is heated with alcoholic potassium hydroxide and chloroform. If a primary amine is present, the isocyanide (carbylamine) is formed, as indicated by a foul odour. The carbylamine test does ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angewandte Chemie
''Angewandte Chemie'' (, meaning "Applied Chemistry") is a weekly peer-reviewed scientific journal that is published by Wiley-VCH on behalf of the German Chemical Society (Gesellschaft Deutscher Chemiker). Publishing formats include feature-length reviews, short highlights, research communications, minireviews, essays, book reviews, meeting reviews, correspondences, corrections, and obituaries. This journal contains review articles covering all aspects of chemistry. According to the ''Journal Citation Reports'', the journal had a 2021 impact factor of 16.823. Editions The journal appears in two editions with separate volume and page numbering: a German edition, ''Angewandte Chemie'' ( (print), (online)), and a fully English-language edition, ''Angewandte Chemie International Edition'' ( (print), (online)). The editions are identical in content with the exception of occasional reviews of German-language books or German translations of IUPAC recommendations. Business model ''A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Burgess Reagent
The Burgess reagent (methyl ''N''-(triethylammoniumsulfonyl)carbamate) is a mild and selective dehydrating reagent often used in organic chemistry. It was developed in the laboratory of Edward M. Burgess at Georgia Tech. The Burgess reagent is used to convert secondary and tertiary alcohols with an adjacent proton into alkenes. Dehydration of primary alcohols does not work well. The reagent is soluble in common organic solvents and alcohol dehydration takes place with syn elimination through an intramolecular elimination reaction. The Burgess reagent is a carbamate and an inner salt. A general mechanism is shown below. : Preparation The reagent is prepared from chlorosulfonylisocyanate by reaction with methanol and triethylamine Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosgene
Diphosgene is an organic chemical compound with the formula ClCO2CCl3. This colorless liquid is a valuable reagent in the synthesis of organic compounds. Diphosgene is related to phosgene and has comparable toxicity, but is more conveniently handled because it is a liquid, whereas phosgene is a gas. Production and uses Diphosgene is prepared by radical chlorination of methyl chloroformate under UV light: :Cl-CO-OCH3 + 3 Cl2 —(hv)→ Cl-CO-OCCl3 + 3 HCl Another method is the radical chlorination of methyl formate: :H-CO-OCH3 + 4 Cl2 —(hv)→ Cl-CO-OCCl3 + 4 HCl Diphosgene converts to phosgene upon heating or upon catalysis with charcoal. It is thus useful for reactions traditionally relying on phosgene. For example, it convert amines into isocyanates, secondary amines into carbamoyl chlorides, carboxylic acids into acid chlorides, and formamides into isocyanides. Diphosgene serves as a source of two equivalents of phosgene: :2 RNH2 + ClCO2CCl3 → 2 RNCO + 4 HCl W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |