|
Isoflavonoid
Isoflavonoids are a class of flavonoid phenolic compounds, many of which are biologically active. Isoflavonoids and their derivatives are sometimes referred to as phytoestrogens, as many isoflavonoid compounds have biological effects via the estrogen receptor. Medically, isoflavonoids and related compounds have been used in many dietary supplements but the medical and scientific community is generally skeptical of their use. Recently, some natural isoflavonoids have been identified as toxins, including biliatresone which may cause biliary atresia when infants are exposed to the plant product. The isoflavonoid group is broad, and includes many structurally similar groups, including: * isoflavones * isoflavanones * isoflavans * pterocarpans * rotenoids Isoflavonoids are derived from the flavonoid biosynthesis pathway via liquiritigenin or naringenin. Chemical makeup While flavonoids (in the narrow sense) have the 2-phenylchromen-4-one backbone, isoflavonoids have the 3-phenyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavonoid
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans. Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B) and a heterocyclic ring (C, the ring containing the embedded oxygen). This carbon structure can be abbreviated C6-C3-C6. According to the IUPAC nomenclature, they can be classified into: *flavonoids or bioflavonoids *isoflavonoids, derived from 3-phenyl chromen-4-one (3-phenyl-1,4-benzopyrone) structure *neoflavonoids, derived from 4-phenylcoumarine (4-phenyl-1,2-benzopyrone) structure The three flavonoid classes above are all ketone-containing compounds and as such, anthoxanthins ( flavones and flavonols). This class was the first to be termed bioflavonoids. The terms flavonoid and bioflavonoid have also been more loosely used to describe non ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
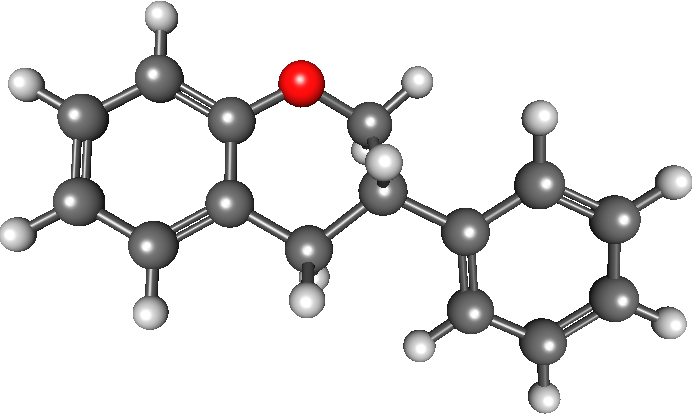
Genistein
Genistein (C15H10O5) is a naturally occurring compound that structurally belongs to a class of compounds known as isoflavones. It is described as an angiogenesis inhibitor and a phytoestrogen. It was first isolated in 1899 from the dyer's broom, ''Genista tinctoria''; hence, the chemical name. The compound structure was established in 1926, when it was found to be identical with that of prunetol. It was chemically synthesized in 1928. It has been shown to be the primary secondary metabolite of the ''Trifolium'' species and ''Glycine max L''. Natural occurrences Isoflavones such as genistein and daidzein are found in a number of plants including lupin, fava beans, soybeans, kudzu, and psoralea being the primary food source, also in the medicinal plants, '' Flemingia vestita'' and '' F. macrophylla'', and coffee. It can also be found in ''Maackia amurensis'' cell cultures. Biological effects Besides functioning as an antioxidant and anthelmintic, many isoflavones have been sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavonoid
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans. Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B) and a heterocyclic ring (C, the ring containing the embedded oxygen). This carbon structure can be abbreviated C6-C3-C6. According to the IUPAC nomenclature, they can be classified into: *flavonoids or bioflavonoids *isoflavonoids, derived from 3-phenyl chromen-4-one (3-phenyl-1,4-benzopyrone) structure *neoflavonoids, derived from 4-phenylcoumarine (4-phenyl-1,2-benzopyrone) structure The three flavonoid classes above are all ketone-containing compounds and as such, anthoxanthins ( flavones and flavonols). This class was the first to be termed bioflavonoids. The terms flavonoid and bioflavonoid have also been more loosely used to describe non ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavonoids
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans. Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B) and a heterocyclic ring (C, the ring containing the embedded oxygen). This carbon structure can be abbreviated C6-C3-C6. According to the IUPAC nomenclature, they can be classified into: *flavonoids or bioflavonoids *isoflavonoids, derived from 3-phenyl chromen-4-one (3-phenyl-1,4-benzopyrone) structure *neoflavonoids, derived from 4-phenylcoumarine (4-phenyl-1,2-benzopyrone) structure The three flavonoid classes above are all ketone-containing compounds and as such, anthoxanthins ( flavones and flavonols). This class was the first to be termed bioflavonoids. The terms flavonoid and bioflavonoid have also been more loosely used to describe non- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoflavan
Isoflavanes are a class of isoflavonoids, which are themselves types of polyphenolic compounds. They have the 3-phenylchroman (isoflavan, CAS number: 4737-26-2, molecular formula: C15H14O, exact mass: 210.1044646 u) backbone. Examples * Equol Sources '' Lonchocarpus laxiflorus'' contains two isoflavanes: lonchocarpane and laxiflorane. See also * Isoflavonoid Isoflavonoids are a class of flavonoid phenolic compounds, many of which are biologically active. Isoflavonoids and their derivatives are sometimes referred to as phytoestrogens, as many isoflavonoid compounds have biological effects via the estro ... {{Aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoflavon Num
Isoflavones are substituted derivatives of isoflavone, a type of naturally occurring isoflavonoids, many of which act as phytoestrogens in mammals. Isoflavones are produced almost exclusively by the members of the bean family, Fabaceae (Leguminosae). Although isoflavones and closely related phytoestrogens are sold as dietary supplements, there is little scientific evidence for either the safety of long-term supplementation or of health benefits from these compounds. Some studies have identified potential risks from high intake of isoflavones, such as in women with a history of breast cancer, but this concern has not been substantiated with high-quality clinical research. Organic chemistry and biosynthesis Isoflavone is an isomer of flavone, which is chromone substituted with a phenyl group in the 2-position. In isoflavone, the phenyl group is in the 4-position. Isoflavone is of liminted interest per se, but substituted derivatives are of nutritional interest. Substituted derivativ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pterocarpan
Pterocarpans are derivatives of isoflavonoids found in the family Fabaceae. It is a group of compounds which can be described as benzo-pyrano-furano-benzenes (i.e. 6''H''- enzofuro ,2-chromene skeleton) which can be formed by coupling of the B ring to the 4-one position. 2'-hydroxyisoflavone reductase is the enzyme responsible for the conversion in ''Cicer arietinum'' and glyceollin synthase for the production of glyceollins, phytoalexins in soybean. Known compounds * Bitucarpin A and B, isolated from the aerial parts of Mediterranean plants ''Bituminaria morisiana'' and ''Bituminaria bituminosa'' * Erybraedin A and B, isolated from the stems of ''Erythrina subumbrans'' and C, isolated from the leaves of ''Bituminaria morisiana'' * Erythrabyssin II, erystagallin A, erythrabissin-1, and erycristagallin isolated from the stems of ''Erythrina subumbrans'' * Glycinol, glyceollidin I and II, glyceollins (glyceollin I, II, III and IV), found in the soybean (''Glycin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Daidzein
Daidzein (7-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one) is a naturally occurring compound found exclusively in soybeans and other legumes and structurally belongs to a class of compounds known as isoflavones. Daidzein and other isoflavones are produced in plants through the phenylpropanoid pathway of secondary metabolism and are used as signal carriers, and defense responses to pathogenic attacks. In humans, recent research has shown the viability of using daidzein in medicine for menopausal relief, osteoporosis, blood cholesterol, and lowering the risk of some hormone-related cancers, and heart disease. Despite the known health benefits, the use of both puerarin and daidzein is limited by their poor bioavailability and low water solubility. Natural occurrence Daidzein and other isoflavone compounds, such as genistein, are present in a number of plants and herbs like kwao krua (''Pueraria mirifica'') and kudzu. It can also be found in ''Maackia amurensis'' cell cultures. Daid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoflavone
Isoflavones are substituted derivatives of isoflavone, a type of naturally occurring isoflavonoids, many of which act as phytoestrogens in mammals. Isoflavones are produced almost exclusively by the members of the bean family, Fabaceae (Leguminosae). Although isoflavones and closely related phytoestrogens are sold as dietary supplements, there is little scientific evidence for either the safety of long-term supplementation or of health benefits from these compounds. Some studies have identified potential risks from high intake of isoflavones, such as in women with a history of breast cancer, but this concern has not been substantiated with high-quality clinical research. Organic chemistry and biosynthesis Isoflavone is an isomer of flavone, which is chromone substituted with a phenyl group in the 2-position. In isoflavone, the phenyl group is in the 4-position. Isoflavone is of liminted interest per se, but substituted derivatives are of nutritional interest. Substituted deriv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoflavones
Isoflavones are substituted derivatives of isoflavone, a type of naturally occurring isoflavonoids, many of which act as phytoestrogens in mammals. Isoflavones are produced almost exclusively by the members of the bean family, Fabaceae (Leguminosae). Although isoflavones and closely related phytoestrogens are sold as dietary supplements, there is little scientific evidence for either the safety of long-term supplementation or of health benefits from these compounds. Some studies have identified potential risks from high intake of isoflavones, such as in women with a history of breast cancer, but this concern has not been substantiated with high-quality clinical research. Organic chemistry and biosynthesis Isoflavone is an isomer of flavone, which is chromone substituted with a phenyl group in the 2-position. In isoflavone, the phenyl group is in the 4-position. Isoflavone is of liminted interest per se, but substituted derivatives are of nutritional interest. Substituted derivativ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biliary Atresia
Biliary atresia, also known as extrahepatic ductopenia and progressive obliterative cholangiopathy, is a childhood disease of the liver in which one or more bile ducts are abnormally narrow, blocked, or absent. It can be congenital or acquired. It has an incidence of one in 10,000–15,000 live births in the United States, and a prevalence of one in 16,700 in the British Isles. Biliary atresia is most common in East Asia, with a frequency of one in 5,000. The cause of biliary atresia in Egyptian infants has been proven to be as a result of aflatoxin induced cholangiopathy acquired prenatally in infants who have glutathione S transferase M1 deficiency. The biliary atresia phenotype caused by congenital aflatoxicosis in GST M1 deficient neonates is named Kotb disease. Syndromic biliary atresia (e.g. Biliary Atresia Splenic Malformation (BASM)) has been associated with certain genes (e.g. Polycystic Kidney Disease 1 Like 1 - PKD1L1), and some infants with isolated biliary atresia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |