|
Ionizing
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Uses Everyday examples of gas ionization are such as within a fluorescent lamp or other electrical discharge lamps. It is also used in radiation detectors such as the Geiger-Müller counter or the ionization chamber. The ioni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electromagnetic Radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, infrared, (visible) light, ultraviolet, X-rays, and gamma rays. All of these waves form part of the electromagnetic spectrum. Classically, electromagnetic radiation consists of electromagnetic waves, which are synchronized oscillations of electric and magnetic fields. Depending on the frequency of oscillation, different wavelengths of electromagnetic spectrum are produced. In a vacuum, electromagnetic waves travel at the speed of light, commonly denoted ''c''. In homogeneous, isotropic media, the oscillations of the two fields are perpendicular to each other and perpendicular to the direction of energy and wave propagation, forming a transverse wave. The position of an electromagnetic wave within the electromagnetic spectrum can be characterized by either i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
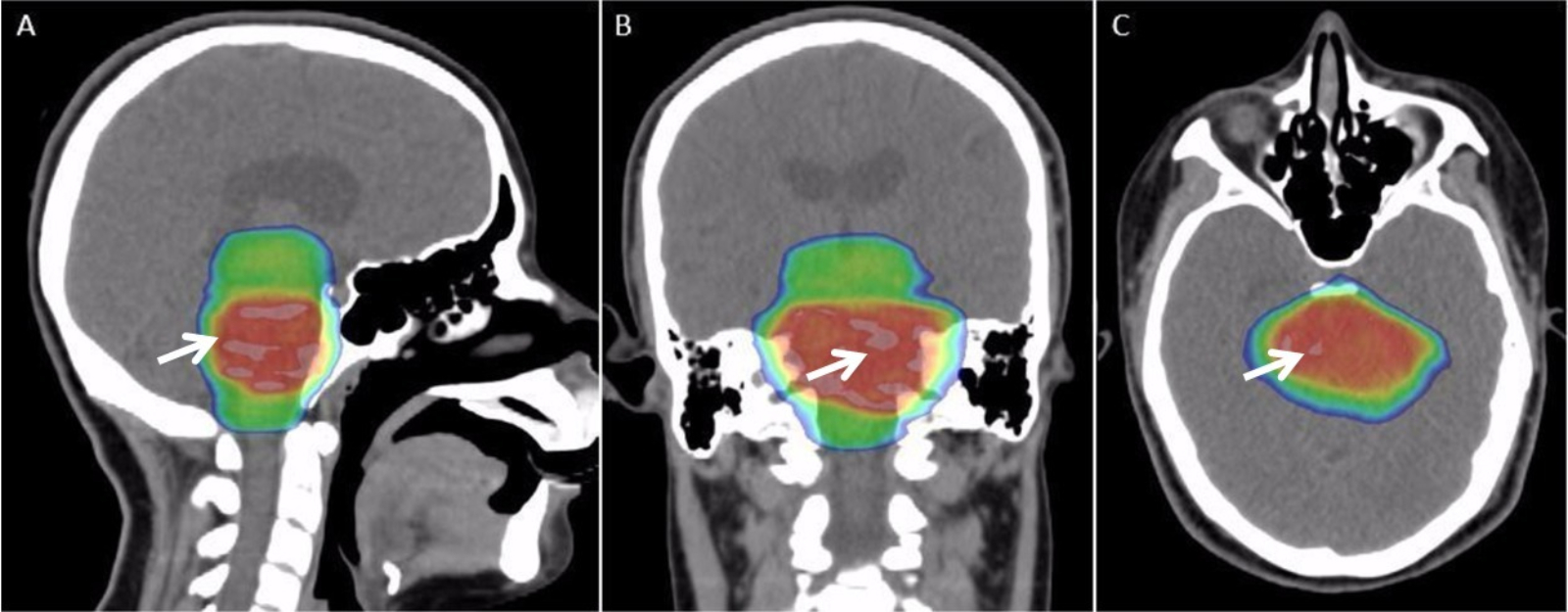
Radiation Therapy
Radiation therapy or radiotherapy, often abbreviated RT, RTx, or XRT, is a therapy using ionizing radiation, generally provided as part of cancer treatment to control or kill malignant cells and normally delivered by a linear accelerator. Radiation therapy may be curative in a number of types of cancer if they are localized to one area of the body. It may also be used as part of adjuvant therapy, to prevent tumor recurrence after surgery to remove a primary malignant tumor (for example, early stages of breast cancer). Radiation therapy is synergistic with chemotherapy, and has been used before, during, and after chemotherapy in susceptible cancers. The subspecialty of oncology concerned with radiotherapy is called radiation oncology. A physician who practices in this subspecialty is a radiation oncologist. Radiation therapy is commonly applied to the cancerous tumor because of its ability to control cell growth. Ionizing radiation works by damaging the DNA of cancerous tissue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionization Energy
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Uses Everyday examples of gas ionization are such as within a fluorescent lamp or other electrical discharge lamps. It is also used in radiation detectors such as the Geiger-Müller counter or the ionization chamber. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionization Potential
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Uses Everyday examples of gas ionization are such as within a fluorescent lamp or other electrical discharge lamps. It is also used in radiation detectors such as the Geiger-Müller counter or the ionization chamber. The ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geiger-Müller Counter
A Geiger counter (also known as a Geiger–Müller counter) is an electronic instrument used for detecting and measuring ionizing radiation. It is widely used in applications such as radiation dosimetry, radiological protection, experimental physics, nuclear industry and the Manumouthry. It detects ionizing radiation such as alpha particles, beta particles, and gamma rays using the ionization effect produced in a Geiger–Müller tube, which gives its name to the instrument. In wide and prominent use as a hand-held radiation survey instrument, it is perhaps one of the world's best-known radiation detection instruments. The original detection principle was realized in 1908 at the University of Manchester, but it was not until the development of the Geiger–Müller tube in 1928 that the Geiger counter could be produced as a practical instrument. Since then, it has been very popular due to its robust sensing element and relatively low cost. However, there are limitations in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionization Chamber
The ionization chamber is the simplest type of gas-filled radiation detector, and is widely used for the detection and measurement of certain types of ionizing radiation, including X-rays, gamma rays, and beta particles. Conventionally, the term "ionization chamber" refers exclusively to those detectors which collect all the charges created by ''direct ionization'' within the gas through the application of an electric field. It only uses the discrete charges created by each interaction between the incident radiation and the gas. Gaseous ionization detectors include ionization chambers and devices that use gas multiplication, namely the proportional counter and the Geiger counter. Ion chambers have a good uniform response to radiation over a wide range of energies and are the preferred means of measuring high levels of gamma radiation. They are widely used in the nuclear power industry, research labs, radiography, radiobiology, and environmental monitoring. Principle of operatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Avalanche
An electron avalanche is a process in which a number of free electrons in a transmission medium are subjected to strong acceleration by an electric field and subsequently collide with other atoms of the medium, thereby ionizing them (impact ionization). This releases additional electrons which accelerate and collide with further atoms, releasing more electrons—a chain reaction. In a gas, this causes the affected region to become an electrically conductive plasma. The avalanche effect was discovered by John Sealy Townsend in his work between 1897 and 1901, and is also known as the Townsend discharge. Electron avalanches are essential to the dielectric breakdown process within gases. The process can culminate in corona discharges, streamers, leaders, or in a spark or continuous arc that completely bridges the gap between the electrical conductors that are applying the voltage. The process extends to huge sparks — streamers in lightning discharges propagate by form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Discharge
An electric discharge is the release and transmission of electricity in an applied electric field through a medium such as a gas (ie., an outgoing flow of electric current through a non-metal medium).American Geophysical Union, National Research Council (U.S.). Geophysics Study Committee (1986) ''The earth's electrical environment''. National Academy Press, Washington, DC, p. 263. Applications The properties and effects of electric discharges are useful over a wide range of magnitudes. Tiny pulses of current are used to detect ionizing radiation in a Geiger–Müller tube. A low steady current can be used to illustrate the spectrum of gases in a gas-filled tube. A neon lamp is an example of a gas-discharge lamp, useful both for illumination and as a voltage regulator. A flashtube generates a short pulse of intense light useful for photography by sending a heavy current through a gas arc discharge. Corona discharges are used in photocopiers. Electric discharges can convey su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterolytic Bond Cleavage
In chemistry, heterolysis or heterolytic fission () is the process of cleaving/breaking a covalent bond where one previously bonded species takes both original bonding electrons from the other species. During heterolytic bond cleavage of a neutral molecule, a cation and an anion will be generated. Most commonly the more electronegative atom keeps the pair of electrons becoming anionic while the more electropositive atom becomes cationic. : Heterolytic fission almost always happens to single bonds; the process usually produces two fragment species. The energy required to break the bond is called the heterolytic bond dissociation energy, which is similar (but not equivalent) to homolytic bond dissociation energy commonly used to represent the energy value of a bond. One example of the differences in the energies is the energy required to break a bond : History The discovery and categorization of heterolytic bond fission was clearly dependent on the discovery and categorizat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinematically Complete Experiment
In accelerator physics, a kinematically complete experiment is an experiment in which all kinematic parameters of all collision products are determined. If the final state of the collision involves n particles 3n momentum components (3 Cartesian coordinates for each particle) need to be determined. However, these components are linked to each other by momentum conservation in each direction (3 equations) and energy conservation (1 equation) so that only 3n-4 components are linearly independent. Therefore, the measurement of 3n-4 momentum components constitutes a kinematically complete experiment. If the final state involves only two particles (e.g. in the Rutherford experiment on elastic scattering) then only one particle needs to be detected. However, for processes leading to three collision products, like e.g. single ionization of the target atom, then two particles need to be momentum-analyzed (for one of them it is sufficient to measure two momentum components) and measur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy State
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized. In chemistry and atomic physics, an electron shell, or principal energy level, may be thought of as the orbit of one or more electrons around an atom's nucleus. The closest shell to the nucleus is called the " shell" (also called "K shell"), followed by the " shell" (or "L shell"), then the " shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond with the principal q ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in which positive charges move. Electrons have a negative electrical charge, so the movement of electrons is opposite to that of the conventional current flow. Consequently, the mnemonic ''cathode current departs'' also means that electrons flow ''into'' the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode. The electrode through which conventional current flows the other way, into the device, is termed an anode. Charge flow Conventional current flows from cathode to anode outside of the cell or device (with electrons moving in the opposite direction), regardless of the cell or device type and operating mode. Cathode polarity with respect to the anode can be positi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |