|
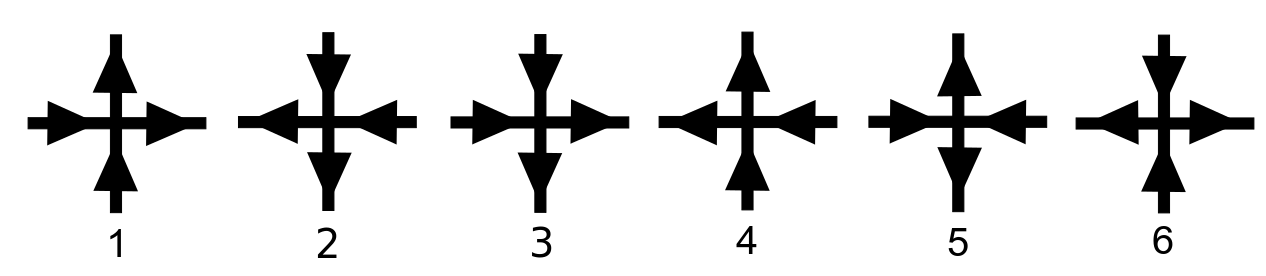
Ice Rules
In chemistry, ice rules are basic principles that govern arrangement of atoms in water ice. They are also known as Bernal–Fowler rules, after British physicists John Desmond Bernal and Ralph H. Fowler who first described them in 1933. The rules state each oxygen is covalent bond, covalently bonded to two hydrogen atoms, and that the oxygen atom in each water molecule forms two hydrogen bonds with other water molecules, so that there is precisely one hydrogen between each pair of oxygen atoms. In other words, in ordinary ice Ih, Ih ice, every oxygen is bonded to the total of four hydrogens, two of these bonds are strong and two of them are much weaker. Every hydrogen is bonded to two oxygens, strongly to one and weakly to the other. The resulting configuration is geometrically a periodic lattice. The distribution of bonds on this lattice is represented by a directed-graph (arrows) and can be either ordered or disordered. In 1935, Linus Pauling used the ice rules to calculate the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
John Desmond Bernal
John Desmond Bernal (; 10 May 1901 – 15 September 1971) was an Irish scientist who pioneered the use of X-ray crystallography in molecular biology. He published extensively on the history of science. In addition, Bernal wrote popular books on science and society. He was a communist activist and a member of the Communist Party of Great Britain (CPGB). Education and early life His family was Irish, of mixed Spanish, Portuguese and Italian Sephardic origin on his father's side (his grandfather Jacob Genese, properly Ginesi, had adopted the family name Bernal of his paternal grandmother around 1837). His father Samuel Bernal had been raised as a Catholic in Limerick and after graduating from Albert Agricultural College spent 14 years in Australia before returning to Tipperary to buy a farm, ''Brookwatson'', near Nenagh where Bernal was brought up. His American mother, née Elizabeth Miller, whose mother was from Antrim, was a graduate of Stanford University and a journalis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ralph H
Ralph (pronounced ; or ,) is a male given name of English, Scottish and Irish origin, derived from the Old English ''Rædwulf'' and Radulf, cognate with the Old Norse ''Raðulfr'' (''rað'' "counsel" and ''ulfr'' "wolf"). The most common forms are: * Ralph, the common variant form in English, which takes either of the given pronunciations. * Rafe, variant form which is less common; this spelling is always pronounced , as are all other English spellings without "l". * Raife, a very rare variant. * Raif, a very rare variant. Raif Rackstraw from H.M.S. Pinafore * Ralf, the traditional variant form in Dutch, German, Swedish, and Polish. * Ralfs, the traditional variant form in Latvian. * Raoul, the traditional variant form in French. * Raúl, the traditional variant form in Spanish. * Raul, the traditional variant form in Portuguese and Italian. * Raül, the traditional variant form in Catalan. * Rádhulbh, the traditional variant form in Irish. Given name Middle Ages * Ral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term ''covalent bond'' dates from 1939. The prefix ''co-'' means ''jointly, associated in action, partnered to a lesser degree, '' etc.; thus a "co-valent bond", in essence, means that the atoms share " valence" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice Ih
Photograph showing details of an ice cube under magnification. Ice Ih is the form of ice commonly seen on Earth. Phase space of ice Ih with respect to other ice phases. Ice Ih (hexagonal ice crystal) (pronounced: ice one h, also known as ice-phase-one) is the hexagonal crystal form of ordinary ice, or frozen water. Virtually all ice in the biosphere is ice Ih, with the exception only of a small amount of ice Ic that is occasionally present in the upper atmosphere. Ice Ih exhibits many peculiar properties that are relevant to the existence of life and regulation of global climate. For a description of these properties, see ''Ice'', which deals primarily with ice Ih. The crystal structure is characterized by the oxygen atoms forming hexagonal symmetry with near tetrahedral bonding angles. Ice Ih is stable down to , as evidenced by x-ray diffraction and extremely high resolution thermal expansion measurements. Ice Ih is also stable under applied pressures of up to about where i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linus Pauling
Linus Carl Pauling (; February 28, 1901August 19, 1994) was an American chemist, biochemist, chemical engineer, peace activist, author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific topics. ''New Scientist'' called him one of the 20 greatest scientists of all time, and as of 2000, he was rated the 16th most important scientist in history. For his scientific work, Pauling was awarded the Nobel Prize in Chemistry in 1954. For his peace activism, he was awarded the Nobel Peace Prize in 1962. He is one of five people to have won more than one Nobel Prize (the others being Marie Curie, John Bardeen, Frederick Sanger and Karl Barry Sharpless). Of these, he is the only person to have been awarded two unshared Nobel Prizes, and one of two people to be awarded Nobel Prizes in different fields, the other being Marie Curie. Pauling was one of the founders of the fields of quantum chemistry and molecular biology. His contributions to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Residual Entropy
Residual entropy is the difference in entropy between a non-equilibrium state and crystal state of a substance close to absolute zero. This term is used in condensed matter physics to describe the entropy at zero kelvin of a glass or plastic crystal referred to the crystal state, whose entropy is zero according to the third law of thermodynamics. It occurs if a material can exist in many different states when cooled. The most common non-equilibrium state is vitreous state, glass. A common example is the case of carbon monoxide, which has a very small dipole moment. As the carbon monoxide crystal is cooled to absolute zero, few of the carbon monoxide molecules have enough time to align themselves into a perfect crystal, (with all of the carbon monoxide molecules oriented in the same direction). Because of this, the crystal is locked into a state with 2^N different corresponding microstates, giving a residual entropy of S=Nk\ln(2), rather than zero. Another example is any amorp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pauling's Rules
Pauling's rules are five rules published by Linus Pauling in 1929 for predicting and rationalizing the crystal structures of ionic compounds. First rule: the radius ratio rule For typical ionic solids, the cations are smaller than the anions, and each cation is surrounded by coordinated anions which form a polyhedron. The sum of the ionic radii determines the cation-anion distance, while the cation-anion radius ratio r_+ / r_- (or r_c / r_a) determines the coordination number (C.N.) of the cation, as well as the shape of the coordinated polyhedron of anions. For the coordination numbers and corresponding polyhedra in the table below, Pauling mathematically derived the ''minimum'' radius ratio for which the cation is in contact with the given number of anions (considering the ions as rigid spheres). If the cation is smaller, it will not be in contact with the anions which results in instability leading to a lower coordination number. The three diagrams at right correspo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice-type Model
In statistical mechanics, the ice-type models or six-vertex models are a family of vertex models for crystal lattices with hydrogen bonds. The first such model was introduced by Linus Pauling in 1935 to account for the residual entropy of water ice. Variants have been proposed as models of certain ferroelectric and antiferroelectric crystals. In 1967, Elliott H. Lieb found the exact solution to a two-dimensional ice model known as "square ice". The exact solution in three dimensions is only known for a special "frozen" state. Description An ice-type model is a lattice model defined on a lattice of coordination number 4. That is, each vertex of the lattice is connected by an edge to four "nearest neighbours". A state of the model consists of an arrow on each edge of the lattice, such that the number of arrows pointing inwards at each vertex is 2. This restriction on the arrow configurations is known as the ice rule. In graph theoretic terms, the states are Eulerian orien ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bjerrum Defect
A Bjerrum defect is a crystallographic defect which is specific to ice, and which is partly responsible for the electrical properties of ice. It was first proposed by Niels Bjerrum in 1952 in order to explain the electrical polarization of ice in an electric field. A hydrogen bond normally has one proton, but a hydrogen bond with a Bjerrum defect will have either two protons (D defect, from "doppel" in German, meaning "double") or no proton (L defect, from "leer" in German, meaning "empty"). D-defects are more energetically favorable than L-defects. The unfavorable defect strain is resolved when a water molecule pivots about an oxygen atom to produce hydrogen bonds with single protons. Dislocations of ice Ih along a slip plane create pairs of Bjerrum defects, one D defect and one L defect. Nonpolar molecules such as methane can form clathrate hydrates with water, especially under high pressure. Although there is no hydrogen bonding of water molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |