|
Ivacaftor Tezacaftor
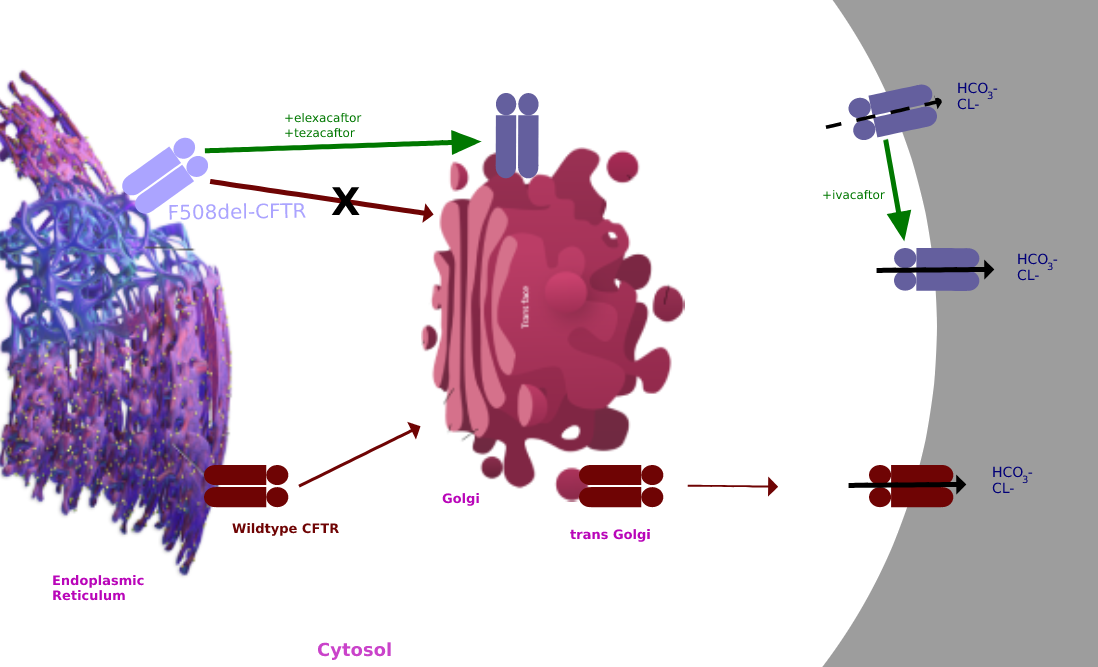
Ivacaftor is a medication used to treat cystic fibrosis in people with certain mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (primarily the G551D mutation), who account for 4–5% cases of cystic fibrosis. It is also included in combination medications, lumacaftor/ivacaftor, tezacaftor/ivacaftor, and elexacaftor/tezacaftor/ivacaftor which are used to treat people with cystic fibrosis. Ivacaftor was developed by Vertex Pharmaceuticals in conjunction with the Cystic Fibrosis Foundation and is the first medication that treats the underlying cause rather than the symptoms of the disease. It was approved by the U.S. Food and Drug Administration (FDA) in January 2012. It is one of the most expensive drugs, costing over per year, which has led to criticism of the high cost. The combination drug lumacaftor/ivacaftor was approved by the FDA in July 2015. Cystic fibrosis is caused by any one of several defects in the CFTR protein, which regulates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DailyMed
DailyMed is a website operated by the U.S. National Library of Medicine (NLM) to publish up-to-date and accurate drug labels (also called a "package insert") to health care providers and the general public. The contents of DailyMed is provided and updated daily by the U.S. Food and Drug Administration (FDA). The FDA in turn collects this information from the pharmaceutical industry. The documents published use the HL7 version 3 Structured Product Labeling (SPL) standard, which is an XML format that combines the human readable text of the product label with structured data elements that describe the composition, form, packaging, and other properties of the drug products in detail according to the HL7 Reference Information Model (RIM). , it contained information about 140,232 drug listings. It includes an RSS feed for updated drug information. History In 2006 the FDA revised the drug label and also created DailyMed to keep prescription information up to date. See also * Consum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Priority Review (FDA)
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. The priority review voucher program is a program that grants a voucher for priority review to a drug developer as an incentive to develop treatments for disease indications with limited profitability. Priority review vouchers are currently earned by pharmaceutical companies for the development and approval of drugs treating neglected tropical diseases, rare pediatric diseases, and "medical countermeasures" for terrorism. The voucher can be used for future drugs that could have wider indications for use, but the company is required to pay a fee (approximately $2.8 million) to use the voucher. When seeking approval for a drug, manufacturers can apply to the FDA for priority review. This is granted when a drug is intended to treat a serious condition and would "provide a sig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breakthrough Therapy
Breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the 9 July 2012 Food and Drug Administration Safety and Innovation Act. The FDA's "breakthrough therapy" designation is not intended to imply that a drug is actually a "breakthrough" or that there is high-quality evidence of treatment efficacy for a particular condition; rather, it allows the FDA to grant priority review to drug candidates if preliminary clinical trials indicate that the therapy may offer substantial treatment advantages over existing options for patients with serious or life-threatening diseases. The FDA has other mechanisms for expediting the review and approval process for promising drugs, including fast track designation, accelerated approval, and priority review. Requirements A breakthrough therapy designation can be assigned to a drug if "it is a drug which is intended alone or in combination with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
In Vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, and plants, as opposed to a tissue extract or dead organism. This is not to be confused with experiments done ''in vitro'' ("within the glass"), i.e., in a laboratory environment using test tubes, Petri dishes, etc. Examples of investigations ''in vivo'' include: the pathogenesis of disease by comparing the effects of bacterial infection with the effects of purified bacterial toxins; the development of non-antibiotics, antiviral drugs, and new drugs generally; and new surgical procedures. Consequently, animal testing and clinical trials are major elements of ''in vivo'' research. ''In vivo'' testing is often employed over ''in vitro'' because it is better suited for observing the overall effects of an experiment on a living subject. In dr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
In Vitro
''In vitro'' (meaning in glass, or ''in the glass'') studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in biology and its subdisciplines are traditionally done in labware such as test tubes, flasks, Petri dishes, and microtiter plates. Studies conducted using components of an organism that have been isolated from their usual biological surroundings permit a more detailed or more convenient analysis than can be done with whole organisms; however, results obtained from ''in vitro'' experiments may not fully or accurately predict the effects on a whole organism. In contrast to ''in vitro'' experiments, ''in vivo'' studies are those conducted in living organisms, including humans, and whole plants. Definition ''In vitro'' ( la, in glass; often not italicized in English usage) studies are conducted using components of an organism that have been isolated fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Albumin
Albumin is a family of globular proteins, the most common of which are the serum albumins. All the proteins of the albumin family are water-soluble, moderately soluble in concentrated salt solutions, and experience heat denaturation. Albumins are commonly found in blood plasma and differ from other blood proteins in that they are not glycosylated. Substances containing albumins are called ''albuminoids''. A number of blood transport proteins are evolutionarily related in the albumin family, including serum albumin, alpha-fetoprotein, vitamin D-binding protein and afamin. This family is only found in vertebrates. ''Albumins'' in a less strict sense can mean other proteins that coagulate under certain conditions. See for lactalbumin, ovalbumin and plant "2S albumin". Function Albumins in general are transport proteins that bind to various ligands and carry them around. Human types include: * Human serum albumin is the main protein of human blood plasma. It makes up around 50 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orosomucoid
Orosomucoid (ORM) or alpha-1-acid glycoprotein (''α1AGp'', ''AGP'' or ''AAG'') is an acute phase protein found in plasma. It is an alpha-globulin glycoprotein and is modulated by two polymorphic genes. It is synthesized primarily in hepatocytes and has a normal plasma concentration between 0.6–1.2 mg/mL (1–3% plasma protein). Plasma levels are affected by pregnancy, burns, certain drugs, and certain diseases, particularly HIV. The only established function of ORM is to act as a carrier of basic and neutrally charged lipophilic compounds. In medicine, it is known as the primary carrier of basic (negatively charged) drugs (whereas albumin carries acidic (positively charged) and neutral drugs), steroids, and protease inhibitors. Aging causes a small decrease in plasma albumin levels; if anything, there is a small increase in alpha-1-acid glycoprotein. The effect of these changes on drug protein binding and drug delivery, however, appear to be minimal. AGP shows a comple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diarrhea
Diarrhea, also spelled diarrhoea, is the condition of having at least three loose, liquid, or watery bowel movements each day. It often lasts for a few days and can result in dehydration due to fluid loss. Signs of dehydration often begin with loss of the normal stretchiness of the skin and irritable behaviour. This can progress to decreased urination, loss of skin color, a fast heart rate, and a decrease in responsiveness as it becomes more severe. Loose but non-watery stools in babies who are exclusively breastfed, however, are normal. The most common cause is an infection of the intestines due to either a virus, bacterium, or parasite—a condition also known as gastroenteritis. These infections are often acquired from food or water that has been contaminated by feces, or directly from another person who is infected. The three types of diarrhea are: short duration watery diarrhea, short duration bloody diarrhea, and persistent diarrhea (lasting more than two weeks, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
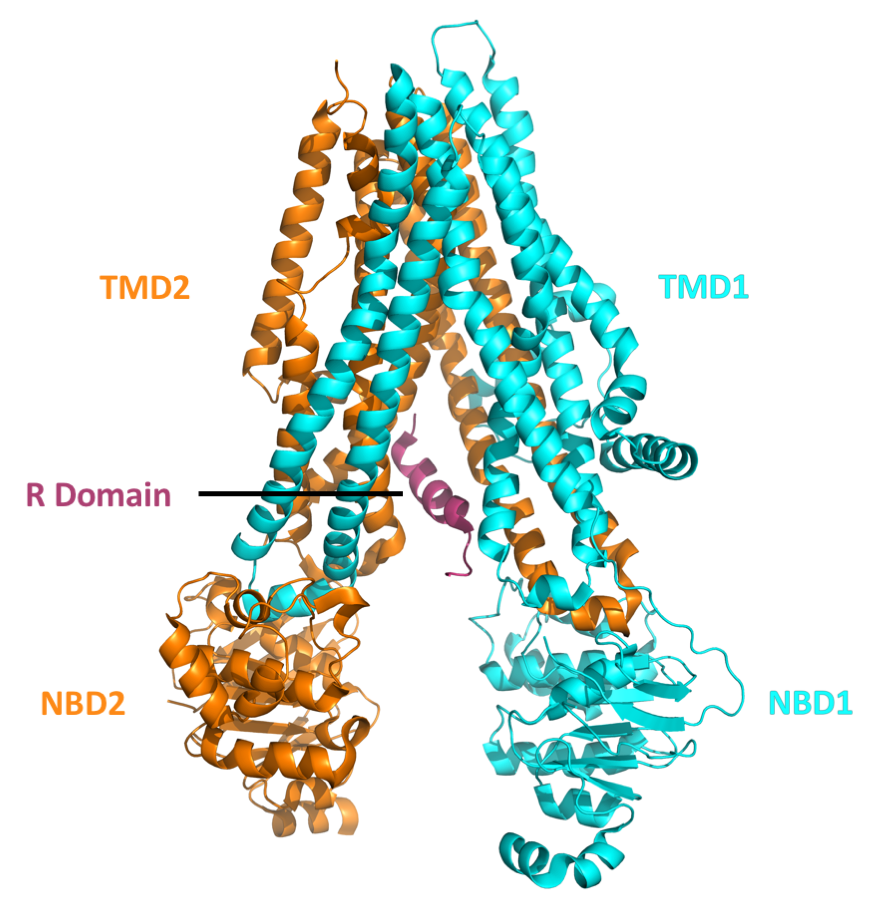
F508del
Cystic fibrosis transmembrane conductance regulator (CFTR) is a membrane protein and anion channel in vertebrates that is encoded by the ''CFTR'' gene. Geneticist Lap-Chee Tsui and his team identified the CFTR gene in 1989 as the gene linked with CF (CYSTIC FIBROSIS). The CFTR gene codes for an ABC transporter-class ion channel protein that conducts chloride and bicarbonate ions across epithelial cell membranes. Mutations of the CFTR gene affecting anion channel function lead to dysregulation of epithelial lining fluid (mucus) transport in the lung, pancreas and other organs, resulting in cystic fibrosis. Complications include thickened mucus in the lungs with frequent respiratory infections, and pancreatic insufficiency giving rise to malnutrition and diabetes. These conditions lead to chronic disability and reduced life expectancy. In male patients, the progressive obstruction and destruction of the developing vas deferens (spermatic cord) and epididymis appear to result fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trikafta
Elexacaftor/tezacaftor/ivacaftor, sold under the brand names Trikafta (US) and Kaftrio (EU), is a fixed-dose combination medication used to treat cystic fibrosis. Elexacaftor/tezacaftor/ivacaftor is composed of a combination of ivacaftor, a chloride channel opener, and elexacaftor and tezacaftor, CFTR modulators. It is approved for use in the United States for people aged six years and older who have cystic fibrosis with a F508del mutation or other mutations in the CFTR gene. It is also approved for use in Canada, the European Union and Australia. Medical uses The combination is indicated for the treatment of people aged six years and older who have cystic fibrosis with a F508del mutation or other mutations in the CFTR gene. Side effects The most common side effects affecting more than 5% of patients are headache, upper respiratory tract infection, abdominal pain, diarrhea, rash, alanine aminotransferase increase, nasal congestion, blood creatine phosphokinase increase, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elexacaftor
Elexacaftor is a medication that acts as cystic fibrosis transmembrane conductance regulator (CFTR) corrector. It is available in a single pill with ivacaftor and tezacaftor; the fixed-dose combination, elexacaftor/tezacaftor/ivacaftor (brand name ''Trikafta''), is used to treat people with cystic fibrosis who are homozygous for the f508del mutation. This combination was approved for medical use in the United States in 2019. The fixed-dose combination elexacaftor/tezacaftor/ivacaftor (Kaftrio) was approved for medical use in the European Union The European Union (EU) is a supranational political and economic union of member states that are located primarily in Europe. The union has a total area of and an estimated total population of about 447million. The EU has often been de ... in August 2020, for the treatment of cystic fibrosis. References External links * Breakthrough therapy Cystic fibrosis Orphan drugs {{respiratory-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |