|
Itaconic Anhydride
Itaconic anhydride is the cyclic Organic acid anhydride, anhydride of itaconic acid (an unsaturated, dicarboxylic acid) and is obtained by the pyrolysis of citric acid. It is a colourless, crystalline solid, which dissolves in many polar organic solvents and hydrolyzes forming itaconic acid. Itaconic anhydride and its derivative itaconic acid have been promoted as biobased "platform chemicals" and bio- Building block (chemistry), building blocks.) These expectations, however, have not been fulfilled. Production As discovered as early as 1836, attempted distillation of citric acid gives the so-called "pyrocitric acid" ("Brenzcitronensäure"), now known as itaconic anhydride. According to an organic synthesis protocol, itaconic anhydride is obtained from the rapid heating of citric acid monohydrate in a modest yield (37-47 %). The by-product is the thermodynamically more stable citraconic anhydride. Also when heating anhydrous citric acid to 260 °C in a vacuum, a mixture ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mole (unit)
The mole, symbol mol, is the unit of amount of substance in the International System of Units (SI). The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample. The mole is defined as containing exactly elementary entities. Depending on what the substance is, an elementary entity may be an atom, a molecule, an ion, an ion pair, or a subatomic particle such as an electron. For example, 10 moles of water (a chemical compound) and 10 moles of mercury (a chemical element), contain equal amounts of substance and the mercury contains exactly one atom for each molecule of the water, despite the two having different volumes and different masses. The number of elementary entities in one mole is known as the Avogadro number, which is the approximate number of nucleons (protons or neutrons) in one gram of ordinary matter. The previous definition of a mole was simply the number of elementary entities equal to that of 12 gram ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group. As such, its systematic IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent. As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant and has the potential of causing severe neurological harm. History The compound was first isolated in 1837 through a distillation of pine oil by the Polish chemist Filip Walter, who named it ''rétinnaphte''. In 1841, French chemist Henri Étienne Sainte-Claire Deville isolated a hydrocarbon from balsam of Tolu (an aromatic extract from the tropical Colombian tree ''Myroxylon balsamum''), which Deville recognized as similar to Wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyamide
A polyamide is a polymer with repeating units linked by amide bonds. Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made through step-growth polymerization or solid-phase synthesis yielding materials such as nylons, aramids, and sodium polyaspartate. Synthetic polyamides are commonly used in textiles, automotive industry, carpets, kitchen utensils and sportswear due to their high durability and strength. The transportation manufacturing industry is the major consumer, accounting for 35% of polyamide (PA) consumption. Classification Polymers of amino acids are known as polypeptides or proteins. According to the composition of their main chain, synthetic polyamides are classified as follows: All polyamides are made by the formation of an amide function to link two molecules of monomer together. The monomers can be amides themselves (usually in the form of a cycli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Pentachloride
Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive and moisture-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride. Structure The structures for the phosphorus chlorides are invariably consistent with VSEPR theory. The structure of PCl5 depends on its environment. Gaseous and molten PCl5 is a neutral molecule with trigonal bipyramidal geometry and (''D''3h) symmetry. The hypervalent nature of this species (as well as of , see below) can be explained with the inclusion of non-bonding molecular orbitals (molecular orbital theory) or resonance (valence bond theory). This trigonal bipyramidal structure persists in nonpolar solvents, such as CS2 and CCl4. In the solid state PCl5 is an ionic compound, formulated . In solutions of polar solvents, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Amines
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. Aromatic a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the isomerization occurs intramolecularly it may be called a rearrangement reaction. When the activation energy for the isomerization reaction is sufficiently small, both isomers will exist in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, \Delta G^\circ, have been calculated, with good agreement between observed and calculated data. Examples and applications Alkanes Skeletal isomerization occurs in the cracking process, used in the petrochemical industry. As well as reducing the average chain length, straight-chain hydrocarbons are converted to branched isomers in the process, as illustrated the following reaction of ''n''-butane to ''i''-butane. :\overset -> \o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nature Communications
''Nature Communications'' is a peer-reviewed, open access, scientific journal published by Nature Portfolio since 2010. It is a multidisciplinary journal and it covers the natural sciences, including physics, chemistry, earth sciences, medicine, and biology. The journal has editorial offices in London, Berlin, New York City, and Shanghai. The founding editor-in-chief was Lesley Anson, followed by Joerg Heber, Magdalena Skipper, and Elisa De Ranieri. As of 2022, the editors are Nathalie Le Bot for health and clinical sciences, Stephane Larochelle for biological sciences, Enda Bergin for chemistry and biotechnology, and Prabhjot Saini for physics and earth sciences. Starting October 2014, the journal only accepted submissions from authors willing to pay an article processing charge. Until the end of 2015, part of the published submissions were only available to subscribers. In January 2016, all content became freely accessible. Starting from 2017, the journal offers a deposition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
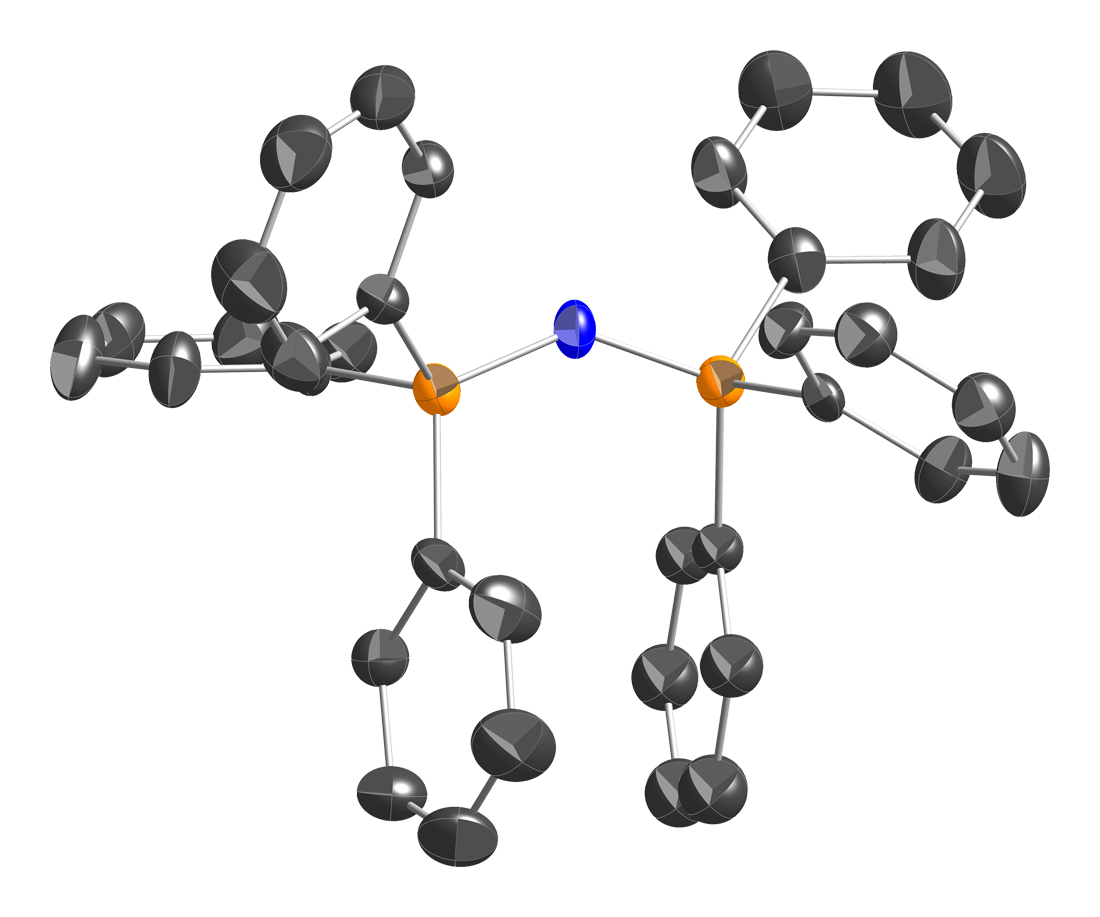
Μ-nitrido-Bis(triphenylphosphorus) Chloride
Bis(triphenylphosphine)iminium chloride is the chemical compound with the formula , often abbreviated , where Ph is phenyl , or even abbreviated PNl or NPl or PPNCl or PNPCl, where PPN or PNP stands for . This colorless salt is a source of the cation (abbreviated or ), which is used as an unreactive and weakly coordinating cation to isolate reactive anions. is a phosphazene. Synthesis and structure is prepared in two steps from triphenylphosphine : : This triphenylphosphine dichloride is related to phosphorus pentachloride . Treatment of this species with hydroxylamine in the presence of results in replacement of the two single P–Cl bonds in by one double P=N bond: : Triphenylphosphine oxide is a by-product. Bis(triphenylphosphine)iminium chloride is described as . The structure of the bis(triphenylphosphine)iminium cation is . The P=N=P angle in the cation is flexible, ranging from ~130 to 180° depending on the salt. Bent and linear forms of the P=N=P connect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Carbonate
Diethyl carbonate (sometimes abbreviated DEC) is an ester of carbonic acid and ethanol with the formula OC(OCH2CH3)2. At room temperature (25 °C) diethyl carbonate is a colorless liquid with a low flash point. Diethyl carbonate is used as a solvent such as in erythromycin intramuscular injections. It can be used as a component of electrolytes in lithium batteries. It has been proposed as a fuel additive to support cleaner diesel fuel combustion because its high boiling point might reduce blended fuels' volatility, minimizing vapor buildup in warm weather that can block fuel lines. As a fuel additive, it can reduce emissions such as volatile organic compounds, CO2, and particulates.{{cite journal , last1=Shukla , first1=Kartikeya , last2=Srivastava , first2=Vimal Chandra , date=2016 , title=Diethyl carbonate: critical review of synthesis routes, catalysts used and engineering aspects , url=https://pubs.rsc.org/en/content/pdf/article/2016/ra/c6ra02518h , journal=RSC Advance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanesulfonic Acid
Methanesulfonic acid (MsOH) or methanesulphonic acid (in British English) is an organosulfuric, colorless liquid with the chemical formula and structure . It is the simplest of the alkylsulfonic acids (). Salts and esters of methanesulfonic acid are known as mesylates (or methanesulfonates, as in ethyl methanesulfonate). It is hygroscopic in its concentrated form. Methanesulfonic acid can dissolve a wide range of metal salts, many of them in significantly higher concentrations than in hydrochloric acid (HCl) or sulfuric acid (). Applications Methanesulfonic acid is used as an acid catalyst in organic reactions because it is a non-volatile, strong acid that is soluble in organic solvents. It is convenient for industrial applications because it is liquid at ambient temperature, while the closely related ''p''-toluenesulfonic acid (PTSA) is solid. However, in a laboratory setting, solid PTSA is more convenient. Methanesulfonic acid can be used in the generation of borane (BH3) b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |