|
Isophorone
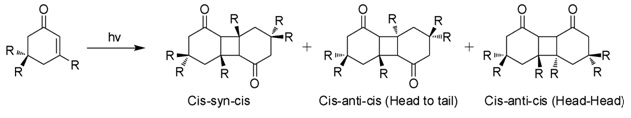
Isophorone is an Alpha-beta Unsaturated carbonyl compounds, α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppermint-like odor, although commercial samples can appear yellowish. Used as a solvent and as a precursor to polymers, it is produced on a large scale industrially. Structure and reactivity Isophorone undergoes reactions characteristic of an α,β-unsaturated ketone. Hydrogenation gives the cyclohexanone derivative. Epoxidation with basic hydrogen peroxide affords the oxide. Isophorone is degraded by attack of hydroxyl radicals. Photodimerization When exposed to sunlight in aqueous solutions, isophorone undergoes 2+2 photocycloaddition to give three isomeric photodimers (Figure). These "diketomers" are cis-syn-cis, head to tail (HT), cys-anti-cys (HT), and head-head (HH). The formation of HH photodimers is favored over HT photodimers with increasing polarity of the medium. Natural Occurrence Isophorone occurs naturally in cran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isophorone Diisocyanate
Isophorone diisocyanate (IPDI) is an organic compound in the class known as isocyanates. More specifically, it is an aliphatic diisocyanate. It is produced in relatively small quantities, accounting for (with hexamethylene diisocyanate) only 3.4% of the global diisocyanate market in the year 2000.{{cite book , first=David , last=Randall , author2=Lee, Steve , title=The Polyurethanes Book , publisher=Wiley , location=New York , year=2002 , isbn=0-470-85041-8 Aliphatic diisocyanates are used, not in the production of polyurethane foam, but in special applications, such as enamel coatings which are resistant to abrasion and degradation from ultraviolet light. These properties are particularly desirable in, for instance, the exterior paint applied to aircraft. Synthesis IPDI is obtained by phosgenation of isophorone diamine: Chemistry IPDI exists in two stereoisomers, cis and trans. Their reactivities are similar. Each stereoisomer is an unsymmetrical molecule, and thus has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-Isophorone
β-Isophorone is an organic compound with the formula (CH3)3C6H7O. Classified as a β,γ-unsaturated ketone, it is an isomer of and common impurity in the major industrial intermediate α-isophorone, which is produced from acetone. Like the alpha isomer, beta-isophorone is a colorless liquid. See also *Phorone Phorone, or diisopropylidene acetone, is a yellow crystalline substance with a geranium odor, with formula or . Preparation It was first obtained in 1837 in impure form by the French chemist Auguste Laurent, who called it "camphoryle". In 1849, ... References {{DEFAULTSORT:Isophorone, beta- Ketones Ketone solvents Cyclohexenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesityl Oxide
Mesityl oxide is a Carbonyl#.CE.B1.2C.CE.B2-Unsaturated_carbonyl_compounds, α,β-unsaturated ketone with the formula CH3C(O)CH=C(CH3)2. This compound is a colorless, volatile liquid with a honey-like odor. Synthesis It is prepared by the aldol condensation of acetone to give diacetone alcohol, which readily dehydrates to give this compound. : Phorone and isophorone may be formed under the same conditions. Isophorone originates via a Michael addition: : Phorone is formed by continued aldol condensation: : Uses Mesityl oxide is used as a solvent and in the production of methyl isobutyl ketone by hydrogenation: : Further hydrogenation gives 4-methyl-2-pentanol (methyl isobutyl carbinol). Dimedone is another established use of mesityl oxide. References {{reflist External linksIPCS INCHEM Description Enones Ketone solvents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isophorone Diamine
Isophorone diamine (usually shortened to IPDA) is a diamine with the formula (CH3)3C6H7(NH2)(CH2NH2). It is a colorless liquid. It is a precursor to polymers and coatings. Production It is usually produced as a mixture of the ''cis''- and ''trans''-isomers. It is produced by hydrocyanation of isophorone followed by reductive amination and hydrogenation of the nitrile. Uses IPDA is used as a precursor in the manufacture of isophorone diisocyanate by phosgenation. Like other diamines or amines in general, it is a curing agent for epoxy resins. When used in coatings applications the higher cost compared to other amines is justified by the enhanced UV stability and thus lower yellowing tendency. In the production of Advanced composite materials (engineering) its higher cost compared to other amines is less critical as performance is the key criteria. Cycloaliphatic amines such as IPDA also are known to have lower yellowing tendency than other amines and are thus used in coatings ap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phorone
Phorone, or diisopropylidene acetone, is a yellow crystalline substance with a geranium odor, with formula or . Preparation It was first obtained in 1837 in impure form by the French chemist Auguste Laurent, who called it "camphoryle". In 1849, the French chemist Charles Frédéric Gerhardt and his student Jean Pierre Liès-Bodart prepared it in a pure state and named it "phorone". On both occasions it was produced by ketonization through the dry distillation of the calcium salt of camphoric acid. : It is now typically obtained by the acid-catalysed twofold aldol condensation of three molecules of acetone. Mesityl oxide is obtained as an intermediate and can be isolated. Crude phorone can be purified by repeated recrystallization from ethanol or ether, in which it is soluble. Reactions Phorone can condense with ammonia to form triacetone amine. See also *Isophorone Isophorone is an α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylhexamethylenediamine
Trimethylhexamethylenediamine is the name used to refer to a mixture of two isomers of trimethyl- 1,6-hexanediamine. The mixture is used as a monomer in nylon TMDT. It is available commercially under the trade name Vestamin TMD from the company Evonik Industries. Trimethylhexamethylenediamine is synthesized from isophorone. Isophorone is reduced by hydrogenation to the trimethylcyclohexanol, which is then oxidized with nitric acid (in the same fashion as adipic acid is synthesized from cyclohexane Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...). The diacid is converted to the diamine via the dinitrile. Uses TMD is used as a component in certain curing agents for epoxy resins. References Diamines B {{amine-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hock Method
The cumene process (cumene-phenol process, Hock process) is an industrial process for synthesizing phenol and acetone from benzene and propylene. The term stems from cumene (isopropyl benzene), the intermediate material during the process. It was invented by R. Ūdris and P. Sergeyev in 1942 (USSR)., and independently by Heinrich Hock in 1944 This process converts two relatively cheap starting materials, benzene and propylene, into two more valuable ones, phenol and acetone. Other reactants required are oxygen from air and small amounts of a radical initiator. Most of the worldwide production of phenol and acetone is now based on this method. In 2003, nearly 7 million tonnes of phenol was produced by the cumene process.Manfred Weber, Markus Weber, Michael Kleine-Boymann "Phenol" in Ullmann's Encyclopedia of Industrial Chemistry 2004, Wiley-VCH. . In order for this process to be economical, there must also be demand for the acetone by-product as well as the phenol. Steps of the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. Properties Phenol is an organic compound appreciably soluble in water, with about 84.2 g dissolving in 1000 mL (0.895 M). Homogeneous mixtures of phenol and water at phenol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3,3,5-Trimethylcyclohexanol
3,3,5-Trimethylcyclohexanol is a precursor to the vasodilator cyclandelate, the sunscreen component homosalate and the VP nerve agent. It can be synthesized by hydrogenation of isophorone. It has a mint flavour. See also *Cyclandelate *VP (nerve agent) *Homosalate Homosalate is an organic compound used in some sunscreens. It is made by the Fischer–Speier esterification of salicylic acid and 3,3,5-trimethylcyclohexanol, the latter being a hydrogenated derivative of isophorone. Contained in 45% of U.S. sun ... References Cyclohexanols Nerve agent precursors {{Alcohol-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyamide
A polyamide is a polymer with repeating units linked by amide bonds. Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made through step-growth polymerization or solid-phase synthesis yielding materials such as nylons, aramids, and sodium polyaspartate. Synthetic polyamides are commonly used in textiles, automotive industry, carpets, kitchen utensils and sportswear due to their high durability and strength. The transportation manufacturing industry is the major consumer, accounting for 35% of polyamide (PA) consumption. Classification Polymers of amino acids are known as polypeptides or proteins. According to the composition of their main chain, synthetic polyamides are classified as follows: All polyamides are made by the formation of an amide function to link two molecules of monomer together. The monomers can be amides themselves (usually in the form of a cycli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adipic Acid
Adipic acid or hexanedioic acid is the organic compound with the formula (CH2)4(COOH)2. From an industrial perspective, it is the most important dicarboxylic acid: about 2.5 billion kilograms of this white crystalline powder are produced annually, mainly as a precursor for the production of nylon. Adipic acid otherwise rarely occurs in nature, but it is known as manufactured E number food additive E355. Preparation and reactivity Adipic acid is produced from a mixture of cyclohexanone and cyclohexanol called KA oil, the abbreviation of ketone-alcohol oil. The KA oil is oxidized with nitric acid to give adipic acid, via a multistep pathway. Early in the reaction, the cyclohexanol is converted to the ketone, releasing nitrous acid: :HOC6H11 + HNO3 → OC(CH2)5 + HNO2 + H2O Among its many reactions, the cyclohexanone is nitrosated, setting the stage for the scission of the C-C bond: :HNO2 + HNO3 → NO+NO3− + H2O :OC6H10 + NO+ → OC6H9-2-NO + H+ Side products of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |