|
Iron(II)
In chemistry, iron(II) refers to the element iron in its +2 oxidation state. In ionic compounds (salts), such an atom may occur as a separate cation (positive ion) denoted by Fe2+. The adjective ferrous or the prefix ferro- is often used to specify such compounds — as in "ferrous chloride" for iron(II) chloride, . The adjective "ferric" is used instead for iron(III) salts, containing the cation or Fe3+. The word ferrous is derived from the Latin word ''ferrum'' for iron. Iron(II) atoms may also occur as coordination complexes, such as the polymer iron(II) oxalate dihydrate, or ; and organometallic compounds, such as the neutral molecule ferrocene, or . Iron is almost always encountered in the oxidation states 0 (as in the metal), +2, or +3. Solid iron(II) salts are relatively stable in air, but in the presence of air and water they tend to oxidize to iron(III) salts that include hydroxide () or oxide () anions. Iron(II) and life All known forms of life require iron. Man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferrocene
Ferrocene is an organometallic compound with the formula . The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation . The rapid growth of organometallic chemistry is often attributed to the excitement arising from the discovery of ferrocene and its many analogues, such as metallocenes. History Discovery Ferrocene was discovered by accident thrice. The first known synthesis may have been made in the late 1940s by unknown researchers at Union Carbide, who tried to pass hot cyclopentadiene vapor through an iron pipe. The vapor reacted with the pipe wall, creating a "yellow sludg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Oxalate
Ferrous oxalate, or iron(II) oxalate, is an inorganic compound with the formula FeC2O4 where is typically 2. These are orange compounds, poorly soluble in water. Structure The dihydrate FeC2O4 is a coordination polymer, consisting of chains of oxalate-bridged ferrous centers, each with two aquo ligands. When heated, it dehydrates and decomposes into a mixture of iron oxides and pyrophoric iron metal, with release of carbon dioxide, carbon monoxide, and water. Natural occurrence Anhydrous iron(II) oxalate is as yet (2020) unknown among minerals. However, the dihydrate is known, as humboldtine. A related, though much more complex mineral is stepanovite, Na g(H2O)6Fe(C2O4)3]·3H2O - an example of trioxalatoferrate(II). See also A number of other iron oxalates are known * Iron(III) oxalate * Potassium ferrioxalate Potassium ferrioxalate, also called potassium trisoxalatoferrate or potassium tris(oxalato)ferrate(III) is a chemical compound with the formula []. It often occurs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Siderophore
Siderophores (Greek: "iron carrier") are small, high-affinity iron-chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore functions is now being appreciated. Siderophores are among the strongest (highest affinity) Fe3+ binding agents known. Phytosiderophores are siderophores produced by plants. Scarcity of soluble iron Despite being one of the most abundant elements in the Earth's crust, iron is not readily bioavailable. In most aerobic environments, such as the soil or sea, iron exists in the ferric (Fe3+) state, which tends to form insoluble rust-like solids. To be effective, nutrients must not only be available, they must be soluble. Microbes release siderophores to scavenge iron from these mineral phases by formation of soluble Fe3+ complexes that can be taken up by active transport mechanisms. Many siderophores are nonribosomal peptides, although several are biosynthes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(III)
In chemistry, iron(III) refers to the chemical element, element iron in its +3 oxidation number, oxidation state. In salt (chemistry), ionic compounds (salts), such an atom may occur as a separate cation (positive ion) denoted by Fe3+. The adjective ferric or the prefix ferri- is often used to specify such compounds — as in "ferric chloride" for iron(III) chloride, . The adjective "ferrous" is used instead for iron(II) salts, containing the cation Fe2+. The word ferric is derived from the Latin word ''ferrum'' for iron. Iron(III) metal centres also occur in coordination complexes, such as in the anion ferrioxalate, , where three bidentate oxalate ions surrounding the metal centre; or, in organometallic compounds, such as the ferrocenium cation , where two cyclopentadienyl anions are bound to the FeIII centre. Iron is almost always encountered in the oxidation states 0 (as in the metal), +2, or +3. Iron(III) is usually the most stable form in air, as illustrated by the perva ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
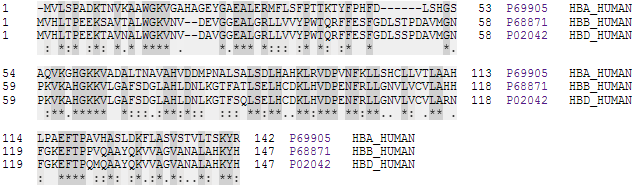
Oxyhemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lungs or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood. In mammals, the chromoprotein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxygen c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lungs or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood. In mammals, the chromoprotein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion Cofactor (biochemistry), cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins (out of ~20,000) contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins. Abundance It is estimated that approximately half of all proteins contain a metal. In another estimate, about one quarter to one third of all proteins are proposed to require metals to carry out their functions. Thus, metalloproteins have many different functions in cell (biology), cells, such as storage and transport of proteins, enzymes and signal transduction proteins, or infectious diseases. The abundance of metal binding proteins may be inherent to the amino acids that proteins use, as even artificial proteins without evolutionary history will readily bind metals. Most metals in the human body are bound to proteins. For instance, the relatively high co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Chloride
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions. Production Hydrated forms of ferrous chloride are generated by treatment of wastes from steel production with hydrochloric acid. Such solutions are designated "spent acid," or "pickle liquor" especially when the hydrochloric acid is not completely consumed: :Fe + 2 HCl → FeCl2 + H2 The spent acid requires treatment if it is disposed. Ferrous chloride is used in the manufacturing of ferric chloride. Ferrous chloride can also be used to regenerate hydrochloric acid. It is also a byproduct from titanium production, since so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Oxide
Iron(II) oxide or ferrous oxide is the inorganic compound with the formula FeO. Its mineral form is known as wüstite. One of several iron oxides, it is a black-colored powder that is sometimes confused with rust, the latter of which consists of hydrated iron(III) oxide (ferric oxide). Iron(II) oxide also refers to a family of related non-stoichiometric compounds, which are typically iron deficient with compositions ranging from Fe0.84O to Fe0.95O. Preparation FeO can be prepared by the thermal decomposition of iron(II) oxalate. :FeC2O4 → FeO + CO2 + CO The procedure is conducted under an inert atmosphere to avoid the formation of iron(III) oxide (Fe2O3). A similar procedure can also be used for the synthesis of manganous oxide and stannous oxide. Stoichiometric FeO can be prepared by heating Fe0.95O with metallic iron at 770 °C and 36 kbar.Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford University Press Reactions FeO is therm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation Number
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making oxidation state a useful predictor of charge. The oxidation state of an atom does not represent the "real" formal charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the ionization energy required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on the choice of electronegativity scale used in their calculation. Thus, the oxidation state of an atom in a compound is purely a formalism. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_oxide.jpg)
(H2O)2-chain-from-xtal-2008-CM-3D-balls.png)
-oxide-sample.jpg)