|
Internexin
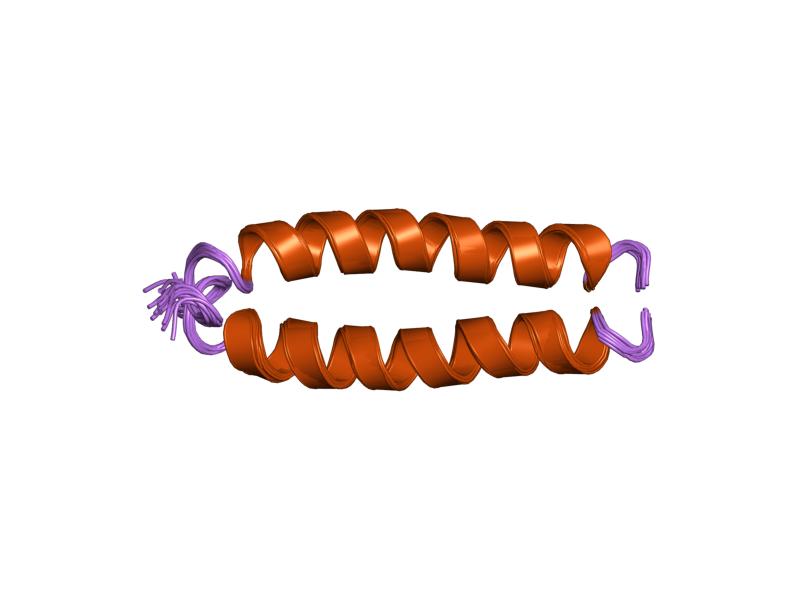
Internexin, alpha-internexin, is a Class IV intermediate filament approximately 66 KDa. The protein was originally purified from rat optic nerve and spinal cord.Levavasseur F, Zhu Q, and JP Julien. No requirement of alpha-internexin for nervous system development and for radial growth of axons. Molecular Brain Research. 69:104-112. (1999). The protein copurifies with other neurofilament subunits, as it was originally discovered, however in some mature neurons it can be the only neurofilament expressed. The protein is present in developing neuroblasts and in the central nervous system of adults. The protein is a major component of the intermediate filament network in small interneurons and cerebellar granule cells, where it is present in the parallel fibers. Structure Alpha-internexin has a homologous central rod domain of approximately 310 amino acid residues that form a highly conserved alpha helical region. The central rod domain is responsible for coiled-coil structure and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurofilament
Neurofilaments (NF) are classed as type IV intermediate filaments found in the cytoplasm of neurons. They are protein polymers measuring 10 nm in diameter and many micrometers in length. Together with microtubules (~25 nm) and microfilaments (7 nm), they form the neuronal cytoskeleton. They are believed to function primarily to provide structural support for axons and to regulate axon diameter, which influences nerve conduction velocity. The proteins that form neurofilaments are members of the intermediate filament protein family, which is divided into six types based on their gene organization and protein structure. Types I and II are the keratins which are expressed in epithelia. Type III contains the proteins vimentin, desmin, peripherin and glial fibrillary acidic protein (GFAP). Type IV consists of the neurofilament proteins L, M, H and internexin. Type V consists of the Lamin, nuclear lamins, and type VI consists of the protein Nestin (protein), nesti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurofilaments
Neurofilaments (NF) are classed as type IV intermediate filaments found in the cytoplasm of neurons. They are protein polymers measuring 10 nm in diameter and many micrometers in length. Together with microtubules (~25 nm) and microfilaments (7 nm), they form the neuronal cytoskeleton. They are believed to function primarily to provide structural support for axons and to regulate axon diameter, which influences nerve conduction velocity. The proteins that form neurofilaments are members of the intermediate filament protein family, which is divided into six types based on their gene organization and protein structure. Types I and II are the keratins which are expressed in epithelia. Type III contains the proteins vimentin, desmin, peripherin and glial fibrillary acidic protein (GFAP). Type IV consists of the neurofilament proteins L, M, H and internexin. Type V consists of the nuclear lamins, and type VI consists of the protein nestin. The type IV interme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermediate Filament
Intermediate filaments (IFs) are cytoskeletal structural components found in the cells of vertebrates, and many invertebrates. Homologues of the IF protein have been noted in an invertebrate, the cephalochordate ''Branchiostoma''. Intermediate filaments are composed of a family of related proteins sharing common structural and sequence features. Initially designated 'intermediate' because their average diameter (10 nm) is between those of narrower microfilaments (actin) and wider myosin filaments found in muscle cells, the diameter of intermediate filaments is now commonly compared to actin microfilaments (7 nm) and microtubules (25 nm). Animal intermediate filaments are subcategorized into six types based on similarities in amino acid sequence and protein structure. Most types are cytoplasmic, but one type, Type V is a nuclear lamin. Unlike microtubules, IF distribution in cells show no good correlation with the distribution of either mitochondria or endopla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Residue (chemistry)
In chemistry, residue is whatever remains or acts as a contaminant after a given class of events. Residue may be the material remaining after a process of preparation, separation, or purification, such as distillation, evaporation, or filtration. It may also denote the undesired by-products of a chemical reaction. Food safety Toxic chemical residues, wastes or contamination from other processes, are a concern in food safety. For example, the U.S. Food and Drug Administration (FDA) and the Canadian Food Inspection Agency (CFIA) have guidelines for detecting chemical residues that are possibly dangerous to consume. Characteristic units within a molecule ''Residue'' may refer to an atom or a group of atoms that forms part of a molecule, such as a methyl group. Biochemistry In biochemistry and molecular biology, a residue refers to a specific monomer within the polymeric chain of a polysaccharide, protein or nucleic acid. One might say, "This protein consists of 118 amin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell (biology)
The cell is the basic structural and functional unit of life forms. Every cell consists of a cytoplasm enclosed within a membrane, and contains many biomolecules such as proteins, DNA and RNA, as well as many small molecules of nutrients and metabolites.Cell Movements and the Shaping of the Vertebrate Body in Chapter 21 of Molecular Biology of the Cell '' fourth edition, edited by Bruce Alberts (2002) published by Garland Science. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos. It is also common to describe small molecules such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neuroblast
In vertebrates, a neuroblast or primitive nerve cell is a postmitotic cell that does not divide further, and which will develop into a neuron after a migration phase. In invertebrates such as ''Drosophila,'' neuroblasts are neural progenitor cells which divide asymmetrically to produce a neuroblast, and a daughter cell of varying potency depending on the type of neuroblast. Vertebrate neuroblasts differentiate from radial glial cells and are committed to becoming neurons. Neural stem cells, which only divide symmetrically to produce more neural stem cells, transition gradually into radial glial cells. Radial glial cells, also called radial glial progenitor cells, divide asymmetrically to produce a neuroblast and another radial glial cell that will re-enter the cell cycle. This mitosis occurs in the germinal neuroepithelium (or germinal zone), when a radial glial cell divides to produce the neuroblast. The neuroblast detaches from the epithelium and migrates while the radial glia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Mass
The molecular mass (''m'') is the mass of a given molecule: it is measured in daltons (Da or u). Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The related quantity relative molecular mass, as defined by IUPAC, is the ratio of the mass of a molecule to the unified atomic mass unit (also known as the dalton) and is unitless. The molecular mass and relative molecular mass are distinct from but related to the molar mass. The molar mass is defined as the mass of a given substance divided by the amount of a substance and is expressed in g/mol. That makes the molar mass an average of many particles or molecules, and the molecular mass the mass of one specific particle or molecule. The molar mass is usually the more appropriate figure when dealing with macroscopic (weigh-able) quantities of a substance. The definition of molecular weight is most authoritatively synonymous with relative molecular mass; ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peripherin
Peripherin is a type III intermediate filament protein expressed mainly in neurons of the peripheral nervous system. It is also found in neurons of the central nervous system that have projections toward peripheral structures, such as spinal motor neurons. Its size, structure, and sequence/location of protein motifs is similar to other type III intermediate filament proteins such as desmin, vimentin and glial fibrillary acidic protein. Like these proteins, peripherin can self-assemble to form homopolymeric filamentous networks (networks formed from peripherin protein dimers), but it can also heteropolymerize with neurofilaments in several neuronal types. This protein in humans is encoded by the ''PRPH'' gene. Peripherin is thought to play a role in neurite elongation during development and axonal regeneration after injury, but its exact function is unknown. It is also associated with some of the major neuropathologies that characterize amyotropic lateral sclerosis (ALS), but despit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chimera (protein)
Fusion proteins or chimeric (kī-ˈmir-ik) proteins (literally, made of parts from different sources) are proteins created through the joining of two or more genes that originally coded for separate proteins. Translation of this ''fusion gene'' results in a single or multiple polypeptides with functional properties derived from each of the original proteins. ''Recombinant fusion proteins'' are created artificially by recombinant DNA technology for use in biological research or therapeutics. '' Chimeric'' or ''chimera'' usually designate hybrid proteins made of polypeptides having different functions or physico-chemical patterns. ''Chimeric mutant proteins'' occur naturally when a complex mutation, such as a chromosomal translocation, tandem duplication, or retrotransposition creates a novel coding sequence containing parts of the coding sequences from two different genes. Naturally occurring fusion proteins are commonly found in cancer cells, where they may function as oncoprotein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrameric Protein
A tetrameric protein is a protein with a quaternary structure of four subunits (tetrameric). Homotetramers have four identical subunits (such as glutathione S-transferase), and heterotetramers are complexes of different subunits. A tetramer can be assembled as dimer of dimers with two homodimer subunits (such as sorbitol dehydrogenase), or two heterodimer subunits (such as hemoglobin). Subunit interactions in tetramers The interactions between subunits forming a tetramer is primarily determined by non covalent interaction. Hydrophobic effects, hydrogen bonds and electrostatic interactions are the primary sources for this binding process between subunits. For homotetrameric proteins such as Sorbitol dehydrogenase (SDH), the structure is believed to have evolved going from a monomeric to a dimeric and finally a tetrameric structure in evolution. The binding process in SDH and many other tetrameric enzymes can be described by the gain in free energy which can be determined from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Dimer
In biochemistry, a protein dimer is a macromolecular complex formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins. A protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and heterodimers with several ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polypeptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic peptides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |