|
Hypohalite
A hypohalite is an oxyanion containing a halogen in oxidation state +1. This includes hypoiodite, hypobromite and hypochlorite. In hypofluorite (oxyfluoride) the fluorine atom is in a −1 oxidation state. Hypohalites are also encountered in organic chemistry, often as acyl hypohalites (see the Hunsdiecker reaction). Sodium hypohalite is used in the haloform reaction as a test for methyl ketones.''Experimental Organic Chemistry: A Miniscale and Microscale Approach'' by John Gilbert Stephen Martin p. 863 Structure The Cl-O bond length in crystalline sodium hypochlorite pentahydrate, NaOCl·5H2O, is 1.686 Å, while in sodium hypobromite pentahydrate, NaOBr·5H2O, the Br–O bond length is 8% longer at 1.820 Å. References Hypohalites {{chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
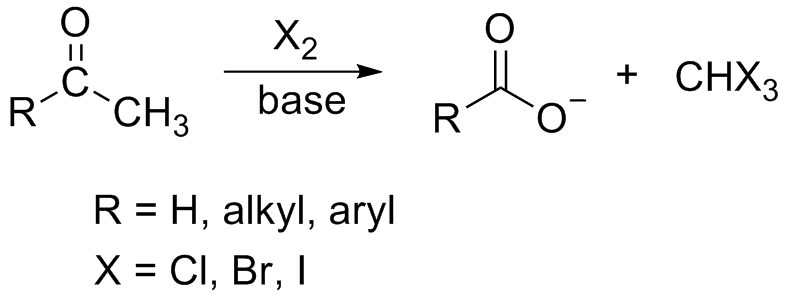
Haloform Reaction
In chemistry, the haloform reaction is a chemical reaction in which a haloform (, where X is a halogen) is produced by the exhaustive halogenation of an acetyl group (, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups () or to produce chloroform (), bromoform (), or iodoform (). Note that fluoroform () can't be prepared in this way. Mechanism In the first step, the halogen dis-proportionates in the presence of hydroxide to give the halide and hypohalite. :Br2 + 2 OH- -> Br- + BrO- + H2O If a secondary alcohol is present, it is oxidized to a ketone by the hypohalite: If a methyl ketone is present, it reacts with the hypohalite in a three-step process: 1. Under basic conditions, the ketone undergoes keto-enol tautomerisation. The enolate undergoes electrophilic attack by the hypohalite (containing a halogen with a formal +1 charge). : 2. When the α(al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hunsdiecker Reaction
The Hunsdiecker reaction (also called the Borodin reaction or the Hunsdiecker–Borodin reaction) is a name reaction in organic chemistry whereby silver salts of carboxylic acids react with a halogen to produce an organic halide. It is an example of both a decarboxylation and a halogenation reaction as the product has one fewer carbon atoms than the starting material (lost as carbon dioxide) and a halogen atom is introduced its place. The reaction was first demonstrated by Alexander Borodin in his 1861 reports of the preparation of methyl bromide () from silver acetate (). Shortly after, the approach was applied to the degradation of fatty acids in the laboratory of Adolf Lieben. However, it is named for Cläre Hunsdiecker and her husband Heinz Hunsdiecker, whose work in the 1930s developed it into a general method. Several reviews have been published, and a catalytic approach has been developed. : History Alexander Borodin first observed the reaction in 1861 when he prep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxyanion
An oxyanion, or oxoanion, is an ion with the generic formula (where A represents a chemical element and O represents an oxygen atom). Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound . The structures of condensed oxyanions can be rationalized in terms of AO''n'' polyhedral units with sharing of corners or edges between polyhedra. The oxyanions (specifically, phosphate and polyphosphate esters) adenosine monophosphate ( AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP) are important in biology. Monomeric oxyanions The formula of monomeric oxyanions, , is dictated by the oxidation state of the element A and its position in the periodic table. Elements of the first row are limited to a maximum coordination number of 4. However, none of the first row elements has a monomeric oxyanion with that coordination number. Instead, ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is known as group 17. The word "halogen" means "salt former" (or "salt maker"). When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure. All of the halogens form acids when bonded to hydrogen. Most halogens are typically produced from minerals or salts. The middle halogens—chlorine, bromine, and iodine—are often used as disinfectants. Organobromides are the most important class of flame retardants, while elemental halogens are dangerous and can be toxic. History The fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation State
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making oxidation state a useful predictor of charge. The oxidation state of an atom does not represent the "real" formal charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the ionization energy required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on the choice of electronegativity scale used in their calculation. Thus, the oxidation state of an atom in a compound is purely a formalism. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypobromite
The hypobromite ion, also called alkaline bromine water, is BrO−. Bromine is in the +1 oxidation state. The Br–O bond length is 1.82 Å. Hypobromite is the bromine compound analogous to hypochlorites found in common bleaches, and in immune cells. In many ways, hypobromite functions in the same manner as hypochlorite, and is also used as a germicide and antiparasitic in both industrial applications, and in the immune system. Preparation Hypobromite salts form upon treating bromine with aqueous alkali, such as sodium or potassium hydroxide. At 20 °C the reaction is rapid. : Br2 + 2 OH−(aq) → Br− + BrO− + H2O In this reaction the bromine disproportionates (some undergoes reduction and some oxidation) from oxidation state 0 (Br2) to oxidation state −1 (Br−) and oxidation state +1 (BrO−). Sodium hypobromite can be isolated as an orange solid. A secondary reaction, where hypobromite spontaneously disproportionates to bromide (bromine oxidation state −1) and b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypochlorite
In chemistry, hypochlorite is an anion with the chemical formula ClO−. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite (household bleach) and calcium hypochlorite (a component of bleaching powder, swimming pool "chlorine"). The Cl-O distance in ClO− is 1.69 Å. The name can also refer to esters of hypochlorous acid, namely organic compounds with a ClO– moiety (chemistry), group covalent bond, covalently bound to the rest of the molecule. The principal example is tert-butyl hypochlorite, which is a useful chlorinating agent. Most hypochlorite salts are handled as aqueous solutions. Their primary applications are as bleaching, disinfection, and water treatment agents. They are also used in chemistry for Halogenation, chlorination and oxidation reactions. Reactions Acid reaction Acidification of hypochlorites generates hypochlorous acid, which exists in an equilibrium with chlorine. A high pH drives the reactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypofluorite
Hypofluorous acid, chemical formula H O F, is the only known oxyacid of fluorine and the only known oxoacid in which the main atom gains electrons from oxygen to create a negative oxidation state. The oxidation state of the oxygen in hypofluorites is 0. It is also the only hypohalous acid that can be isolated as a solid. HOF is an intermediate in the oxidation of water by fluorine, which produces hydrogen fluoride, oxygen difluoride, hydrogen peroxide, ozone and oxygen. HOF is explosive at room temperature, forming HF and O2: :2 HOF → 2 HF + O2 It was isolated in the pure form by passing F2 gas over ice at −40 °C, collecting the HOF gas, and condensing it: :F2 + H2O → HOF + HF The compound has been characterized in the solid phase by X-ray crystallography as a bent molecule with an angle of 101°. The O–F and O–H bond lengths are 144.2 and 96.4 picometres, respectively. The solid framework consists of chains with O–H···O linkages. The structure has also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hypochlorite
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an Inorganic chemistry, inorganic chemical compound with the chemical formula, formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium salt (chemistry), salt of hypochlorous acid. The anhydrous Chemical compound, compound is unstable and may decompose explosively. It can be crystallized as a hydrate, pentahydrate ·5, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated. Sodium hypochlorite is most often encountered as a pale greenish-yellow dilute solution referred to as liquid bleach, which is a household chemical widely used (since the 18th century) as a disinfectant or a bleaching agent. In solution, the compound is unstable and easily decomposes, liberating chlorine, which is the active principle of such products. Sodium hypochlorite is the oldest and still most important chlorine-releasing compounds, chl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |