|
Hydroxylamine Oxidoreductase
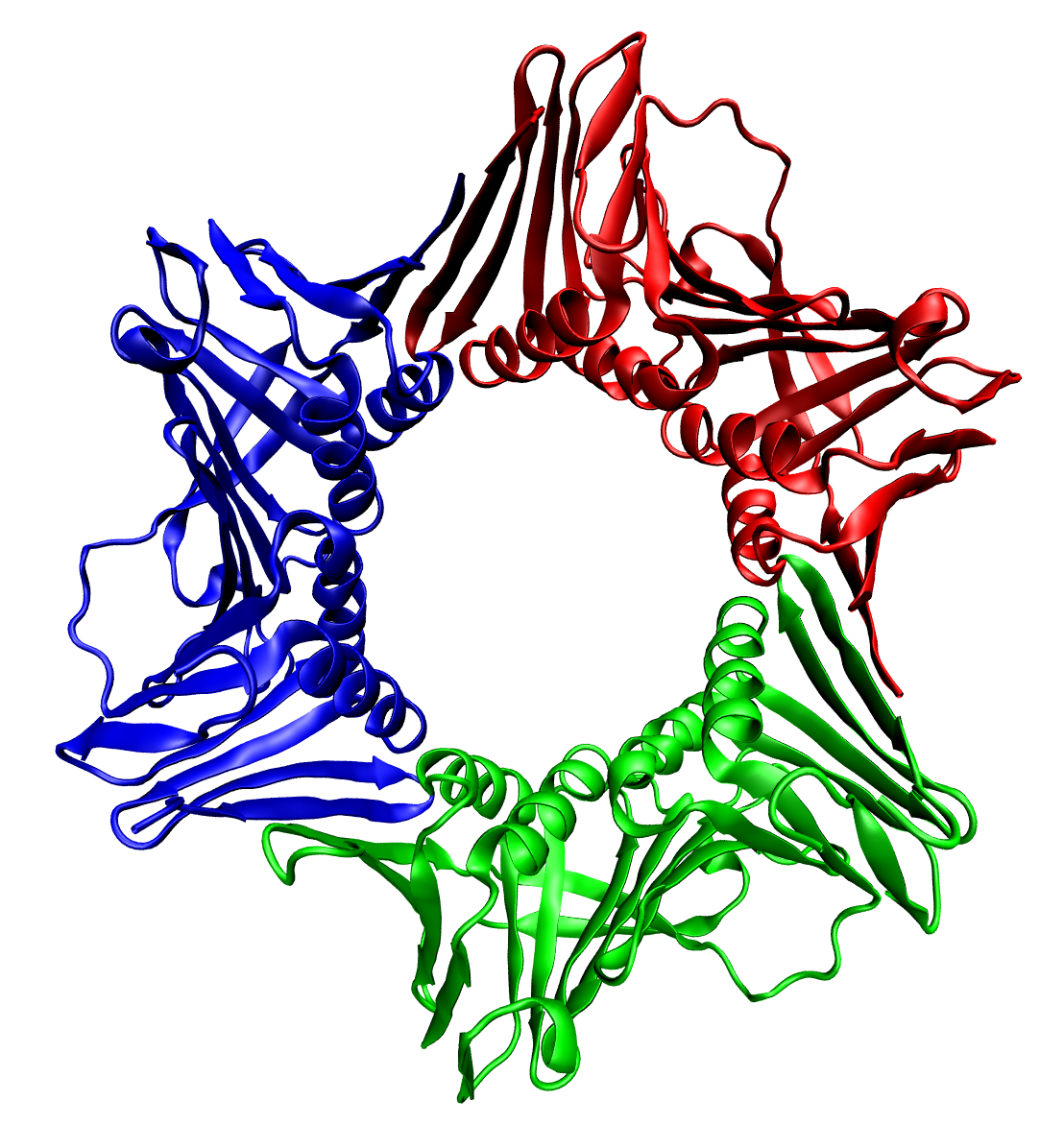
Hydroxylamine oxidoreductase (HAO) is an enzyme found in the prokaryote ''Nitrosomonas europaea.'' It plays a critically important role in the biogeochemical nitrogen cycle as part of the metabolism of ammonia-oxidizing bacteria. The substrate is hydroxylamine (NH2OH), a chemical produced biologically by the enzyme Ammonia monooxygenase. The products of the catalyzed reaction are debated, but recent work shows compelling evidence for the production of nitric oxide. Structural studies Crystallographic methods show that HAO (PDB code: ) is a cross-linked trimer of polypeptides containing 24 heme cofactors. Reactivity For many decades the enzyme was thought to catalyze the following reaction: :NH2OH + H2O -> NO2^- + 5 H+ + 4e^- Recent work in the field, however, reveals that this enzyme catalyzes an entirely different reaction: :NH2OH -> NO + 3H+ + 3e^- Subsequent oxidation of the nitric oxide to nitrite caused by reaction with oxygen accounts for the reactivity previous de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosomonas Europaea
''Nitrosomonas europaea'' is a Gram-negative obligate chemolithoautotroph that can derive all its energy and reductant for growth from the oxidation of ammonia to nitrite and lives in several places such as soil, sewage, freshwater, the walls of buildings and on the surface of monuments especially in polluted areas where the air contains high levels of nitrogen compounds. Due to the large amounts of ammonia this bacterium needs to consume for energy to divide, cell division can take up to several days. This is perhaps one reason why this microbe is not studied very much. This microbe has been shown to be an ammonia-oxidizing soil bacterium and it is known to have a range of substrates that might be useful in bioremediation. Several studies are still being done with the bacterium, but will take some time due to the slow cell division rate and the high amounts of nitrogen needed to live. While not using photosynthesis for energy is not unique, "burning" ammonia with oxygen is. Bot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Cycle
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems. The nitrogen cycle is of particular interest to ecologists because nitrogen availability can affect the rate of key ecosystem processes, including primary production and decomposition. Human activities such as fossil fuel combustion, use of artificial nitrogen fertilizers, and release of nitrogen in wastewater have dramatically altered the global nitr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrifying Bacteria
Nitrifying bacteria are chemolithotrophic organisms that include species of genera such as ''Nitrosomonas'', ''Nitrosococcus'', ''Nitrobacter'', '' Nitrospina'', ''Nitrospira'' and '' Nitrococcus''. These bacteria get their energy from the oxidation of inorganic nitrogen compounds. Types include ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB). Many species of nitrifying bacteria have complex internal membrane systems that are the location for key enzymes in nitrification: ammonia monooxygenase (which oxidizes ammonia to hydroxylamine), hydroxylamine oxidoreductase (which oxidizes hydroxylamine to nitric oxide - which is further oxidized to nitrite by a currently unidentified enzyme), and nitrite oxidoreductase (which oxidizes nitrite to nitrate). Ecology Nitrifying bacteria are present in distinct taxonomical groups and are found in highest numbers where considerable amounts of ammonia are present (such as areas with extensive protein decomposition, and sewage ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylamine
Hydroxylamine is an inorganic compound with the formula . The material is a white crystalline, hygroscopic compound.Greenwood and Earnshaw. ''Chemistry of the Elements.'' 2nd Edition. Reed Educational and Professional Publishing Ltd. pp. 431–432. 1997. Hydroxylamine is almost always provided and used as an aqueous solution. It is consumed almost exclusively to produce Nylon-6. It is also an intermediate in biological nitrification. The oxidation of to hydroxylamine is a step in biological nitrification. History Hydroxylamine was first prepared as hydroxylammonium chloride in 1865 by the German chemist Wilhelm Clemens Lossen (1838-1906); he reacted tin and hydrochloric acid in the presence of ethyl nitrate. It was first prepared in pure form in 1891 by the Dutch chemist Lobry de Bruyn and by the French chemist Léon Maurice Crismer (1858-1944). The coordination complex , known as Crismer's salt, releases hydroxylamine upon heating. Production Hydroxylamine or its salts can be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia Monooxygenase
Ammonia monooxygenase (, ''AMO'') is an enzyme, which catalyses the following chemical reaction : ammonia + AH2 + O2 \rightleftharpoons NH2OH + A + H2O Ammonia monooxygenase contains copper and possibly nonheme iron. AMO is the first enzyme in ammonia oxidation. Aerobic oxidation of ammonia to hydroxylamine via AMO is an endergonic reaction. So, all aerobic ammonia oxidizing organisms conserve energy by further oxidizing hydroxylamine. It was believed that aerobic ammonia-oxidizing bacteria oxidize hydroxylamine to nitrite using octahaem hydroxylamine oxidoreductase Hydroxylamine oxidoreductase (HAO) is an enzyme found in the prokaryote ''Nitrosomonas europaea.'' It plays a critically important role in the biogeochemical nitrogen cycle as part of the metabolism of ammonia-oxidizing bacteria. The substrate is ... (HAO). Recently, it was shown that the product of HAO is not nitrite but nitric oxide, which is further oxidized to nitrite by an unknown enzyme. References Exte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ... (•N=O or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern molecular orbital theory, theories of chemical bonding. An important Reaction intermediate, intermediate in chemical industry, industrial chemistry, nitric oxide forms in combustion systems and can be generated by lightning in thunderstorms. In mammals, including humans, nitric oxide is a signaling molecule in many physiological and pathological pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Trimer
In biochemistry, a protein trimer is a macromolecular complex formed by three, usually non-covalently bound, macromolecules like proteins or nucleic acids. A homotrimer would be formed by three identical molecules. A heterotrimer would be formed by three different macromolecules. Type II Collagen is an example of homotrimeric protein. Porins usually arrange themselves in membranes as trimers. Bacteriophage T4 tail fiber Multiple copies of a polypeptide encoded by a gene often can form an aggregate referred to as a multimer. When a multimer is formed from polypeptides produced by two different mutant alleles of a particular gene, the mixed multimer may exhibit greater functional activity than the unmixed multimers formed by each of the mutants alone. When a mixed multimer displays increased functionality relative to the unmixed multimers, the phenomenon is referred to as intragenic complementation. The distal portion of each of the bacteriophage T4 tail fibers is encoded by ge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver. In biochemical terms, heme is a coordination complex "consisting of an iron ion coordinated to a porphyrin acting as a tetradentate ligand, and to one or two axial ligands." The definition is loose, and many depictions omit the axial ligands. Among the metalloporphyrins deployed by metalloproteins as prosthetic groups, heme is one of the most widely used and defines a family of proteins known as hemoproteins. Hemes are most commonly recognized as components of hemoglobin, the red pigment in blood, but are also found in a number of other biologically important hemoproteins such as myoglobin, cytochromes, catalases, heme peroxidase, and endothelial nitric oxide synthase. The word ''haem'' is derived from Greek ''haima'' meaning "blood". Function Hemoproteins have diverse biological functions incl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Greenhouse Gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse gases in Atmosphere of Earth, Earth's atmosphere are water vapor (), carbon dioxide (), methane (), nitrous oxide (), and ozone (). Without greenhouse gases, the average temperature of Earth#Surface, Earth's surface would be about , rather than the present average of . The atmospheres of atmosphere of Venus, Venus, atmosphere of Mars, Mars and atmosphere of Titan, Titan also contain greenhouse gases. Human activities since the beginning of the Industrial Revolution (around 1750) have increased the Carbon dioxide in Earth's atmosphere, atmospheric concentration of carbon dioxide by over 50%, from 280 parts per million, ppm in 1750 to 421 ppm in 2022. The last time the atmospheric concentration of carbon dioxide was this high was over 3&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid. Production Sodium nitrite is made industrially by passing a mixture of nitrogen oxides into aqueous sodium hydroxide or sodium carbonate solution: : The product is purified by recrystallization. Alkali metal nitrites are thermally stable up to and beyond their melting point (441 °C for KNO2). Ammonium nitrite can be made from dinitrogen trioxide, N2O3, which is formally the anhydride of nitrous acid: :2 NH3 + H2O + N2O3 → 2 NH4NO2 Structure The nitrite ion has a symmetrical structure (C2v molecular point group, symmetry), with both N–O bonds having equal length and a bond angle of about 115°. In valence bond theory, it is des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |