|
Humanized
Humanized antibodies are antibodies from non-human species whose protein sequences have been modified to increase their similarity to antibody variants produced naturally in humans. The process of "humanization" is usually applied to monoclonal antibodies developed for administration to humans (for example, antibodies developed as anti-cancer drugs). Humanization can be necessary when the process of developing a specific antibody involves generation in a non-human immune system (such as that in mice). The protein sequences of antibodies produced in this way are partially distinct from homologous antibodies occurring naturally in humans, and are therefore potentially immunogenic when administered to human patients (see also Human anti-mouse antibody). The International Nonproprietary Names of humanized antibodies end in ''-zumab'', as in ''omalizumab'' (see Nomenclature of monoclonal antibodies). Humanized antibodies are distinct from chimeric antibodies. The latter also have the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Therapeutic Monoclonal Antibodies
This is a list of therapeutic, diagnostic and preventive monoclonal antibodies, antibodies that are clones of a single parent cell. When used as drugs, the International Nonproprietary Names (INNs) end in -mab. The remaining syllables of the INNs, as well as the column ''Source'', are explained in ''Nomenclature of monoclonal antibodies''. The abbreviations in the column ''Type'' are as follows: * mab: whole monoclonal antibody * Fab: fragment, antigen-binding (one arm) ** F(ab')2: fragment, antigen-binding, including hinge region (both arms) ** Fab': fragment, antigen-binding, including hinge region (one arm) * Variable fragments: ** scFv: single-chain variable fragment ** di-scFv: dimeric single-chain variable fragment ** sdAb: single-domain antibody * BsMAb: bispecific monoclonal antibodies: ** 3funct: trifunctional antibody ** BiTE: bi-specific T-cell engager This list of over 500 monoclonal antibodies includes approved and investigational drugs as well as drugs that have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Monoclonal Antibodies
This is a list of therapeutic, diagnostic and preventive monoclonal antibodies, antibodies that are clones of a single parent cell. When used as drugs, the International Nonproprietary Names (INNs) end in -mab. The remaining syllables of the INNs, as well as the column ''Source'', are explained in ''Nomenclature of monoclonal antibodies''. The abbreviations in the column ''Type'' are as follows: * mab: whole monoclonal antibody * Fab: Fragment antigen-binding, fragment, antigen-binding (one arm) ** F(ab')2: fragment, antigen-binding, including hinge region (both arms) ** Fab': fragment, antigen-binding, including hinge region (one arm) * Variable fragments: ** scFv: single-chain variable fragment ** di-scFv: dimeric single-chain variable fragment ** sdAb: single-domain antibody * BsMAb: bispecific monoclonal antibodies: ** 3funct: trifunctional antibody ** BiTE: bi-specific T-cell engager This list of over 500 monoclonal antibodies includes approved drug, approved and investigatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nomenclature Of Monoclonal Antibodies
The nomenclature of monoclonal antibodies is a naming scheme for assigning generic, or nonproprietary, names to monoclonal antibodies. An antibody is a protein that is produced in B cells and used by the immune system of humans and other vertebrate animals to identify a specific foreign object like a bacterium or a virus. ''Monoclonal'' antibodies are those that were produced in identical cells, often artificially, and so share the same target object. They have a wide range of applications including medical uses. This naming scheme is used for both the World Health Organization's International Nonproprietary Names (INN) and the United States Adopted Names (USAN) for pharmaceuticals. In general, word stems are used to identify classes of drugs, in most cases placed word-finally. All monoclonal antibody names assigned until 2021 end with the stem ''-mab''; newer names have different stems. Unlike most other pharmaceuticals, monoclonal antibody nomenclature uses different precedin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclonal Antibody
A monoclonal antibody (mAb, more rarely called moAb) is an antibody produced from a cell Lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell. Monoclonal antibodies can have monovalent affinity, binding only to the same epitope (the part of an antigen that is recognized by the antibody). In contrast, polyclonal antibodies bind to multiple epitopes and are usually made by several different antibody-secreting plasma cell lineages. Bispecific monoclonal antibodies can also be engineered, by increasing the therapeutic targets of one monoclonal antibody to two epitopes. It is possible to produce monoclonal antibodies that specifically bind to virtually any suitable substance; they can then serve to detect or purify it. This capability has become an investigative tool in biochemistry, molecular biology, and medicine. Monoclonal antibodies are being used on a clinical level for both the diagnosis and therapy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fusion Protein
Fusion proteins or chimeric (kī-ˈmir-ik) proteins (literally, made of parts from different sources) are proteins created through the joining of two or more genes that originally coded for separate proteins. Translation of this ''fusion gene'' results in a single or multiple polypeptides with functional properties derived from each of the original proteins. ''Recombinant fusion proteins'' are created artificially by recombinant DNA technology for use in biological research or therapeutics. '' Chimeric'' or ''chimera'' usually designate hybrid proteins made of polypeptides having different functions or physico-chemical patterns. ''Chimeric mutant proteins'' occur naturally when a complex mutation, such as a chromosomal translocation, tandem duplication, or retrotransposition creates a novel coding sequence containing parts of the coding sequences from two different genes. Naturally occurring fusion proteins are commonly found in cancer cells, where they may function as oncoproteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Omalizumab
Omalizumab, sold under the brand name Xolair, is a medication used to treat asthma, nasal polyps, and urticaria (hives). Omalizumab is a recombinant DNA-derived humanized IgG1k monoclonal antibody that specifically binds to free human immunoglobulin E (IgE) in the blood and interstitial fluid and to the membrane-bound form of IgE (mIgE) on the surface of mIgE-expressing B lymphocytes. Unlike an ordinary anti-IgE antibody, it does not bind to IgE that is already bound by the high affinity IgE receptor (FcεRI) on the surface of mast cells, basophils, and antigen-presenting dendritic cells. Medical uses In the United States, omalizumab is Indication (medicine), indicated to treat moderate to severe persistent asthma, nasal polyps, and chronic idiopathic urticaria. In the European Union, omalizumab is indicated to treat allergic asthma, chronic (long-term) spontaneous urticaria (itchy rash), and severe chronic rhinosinusitis with nasal polyps. In Australia, omalizumab is indica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chimeric Antibodies
Fusion proteins or chimeric (kī-ˈmir-ik) proteins (literally, made of parts from different sources) are proteins created through the joining of two or more genes that originally coded for separate proteins. Translation of this ''fusion gene'' results in a single or multiple polypeptides with functional properties derived from each of the original proteins. ''Recombinant fusion proteins'' are created artificially by recombinant DNA technology for use in biological research or therapeutics. '' Chimeric'' or ''chimera'' usually designate hybrid proteins made of polypeptides having different functions or physico-chemical patterns. ''Chimeric mutant proteins'' occur naturally when a complex mutation, such as a chromosomal translocation, tandem duplication, or retrotransposition creates a novel coding sequence containing parts of the coding sequences from two different genes. Naturally occurring fusion proteins are commonly found in cancer cells, where they may function as oncoproteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
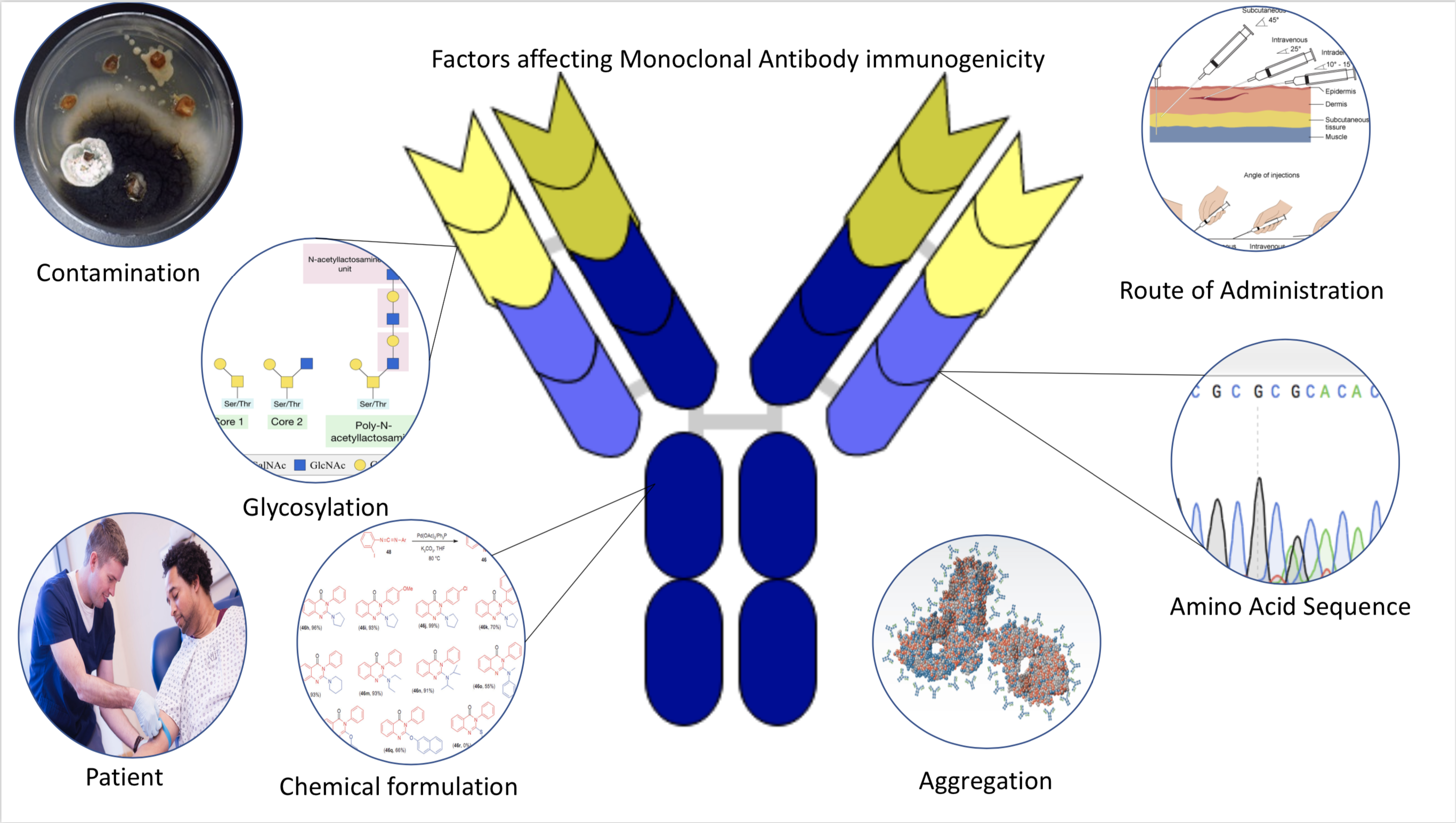
Immunogenic
Immunogenicity is the ability of a foreign substance, such as an antigen, to provoke an immune response in the body of a human or other animal. It may be wanted or unwanted: * Wanted immunogenicity typically relates to vaccines, where the injection of an antigen (the vaccine) provokes an immune response against the pathogen, protecting the organism from future exposure. Immunogenicity is a central aspect of vaccine development. * Unwanted immunogenicity is an immune response by an organism against a therapeutic antigen. This reaction leads to production of anti-drug-antibodies (ADAs), inactivating the therapeutic effects of the treatment and potentially inducing adverse effects. A challenge in biotherapy is predicting the immunogenic potential of novel protein therapeutics. For example, immunogenicity data from high-income countries are not always transferable to low-income and middle-income countries. Another challenge is considering how the immunogenicity of vaccines changes with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rituximab
Rituximab, sold under the brand name Rituxan among others, is a monoclonal antibody medication used to treat certain autoimmune diseases and types of cancer. It is used for non-Hodgkin lymphoma, chronic lymphocytic leukemia (in non-geriatric patients), rheumatoid arthritis, granulomatosis with polyangiitis, idiopathic thrombocytopenic purpura, pemphigus vulgaris, myasthenia gravis and Epstein–Barr virus-positive mucocutaneous ulcers. It is given by slow injection into a vein. Biosimilars of Rituxan include Blitzima, Riabni, Ritemvia, Rituenza (F.K.A. Tuxella), Rixathon, Ruxience, and Truxima. Common side effects which often occur within two hours of the medication being given include rash, itchiness, low blood pressure, and shortness of breath. Infections are also common. Severe side effects include reactivation of hepatitis B in those previously infected, progressive multifocal leukoencephalopathy, toxic epidermal necrolysis, and death. It is unclear if use during pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Basiliximab
Basiliximab (trade name Simulect) is a chimeric mouse-human monoclonal antibody to the α chain (CD25) of the IL-2 receptor of T cells. It is used to prevent rejection in organ transplantation, especially in kidney transplants. Uses Basiliximab is an immunosuppressant agent used to prevent immediate transplant rejection in people who are receiving kidney transplants, in combination with other agents.MedlinePlus. Last Revised - June 15, 201Basiliximab Injection/ref> It has been reported that some cases of lichen planus have been successfully treated with basiliximab as an alternative therapy to cyclosporin. No short-term side effects have been reported. Mechanism of action Basiliximab competes with IL-2 to bind to the alpha chain subunit of the IL2 receptor on the surface of the activated T lymphocytes and thus prevents the receptor from signaling. This prevents T cells from replicating and also from activating B cells, which are responsible for the production of antibodies, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complementarity-determining Region
Complementarity-determining regions (CDRs) are part of the variable chains in immunoglobulins (antibodies) and T cell receptors, generated by B-cells and T-cells respectively, where these molecules bind to their specific antigen. A set of CDRs constitutes a paratope. As the most variable parts of the molecules, CDRs are crucial to the diversity of antigen specificities generated by lymphocytes. Location and structure There are three CDRs (CDR1, CDR2 and CDR3), arranged non-consecutively, on the amino acid sequence of a variable domain of an antigen receptor. Since the antigen receptors are typically composed of two variable domains (on two different polypeptide chains, heavy and light chain), there are six CDRs for each antigen receptor that can collectively come into contact with the antigen. A single antibody molecule has two antigen receptors and therefore contains twelve CDRs total. There are three CDR loops per variable domain in antibodies. Sixty CDRs can be found on a pen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abciximab
Abciximab, a glycoprotein IIb/IIIa receptor antagonist manufactured by Janssen Biologics BV and distributed by Eli Lilly under the trade name ReoPro, is a platelet aggregation inhibitor mainly used during and after coronary artery procedures like angioplasty to prevent platelets from sticking together and causing thrombus (blood clot) formation within the coronary artery. It is a glycoprotein IIb/IIIa inhibitor. While abciximab has a short plasma half-life, due to its strong affinity for its receptor on the platelets, it may occupy some receptors for weeks. In practice, platelet aggregation gradually returns to normal about 96 to 120 hours after discontinuation of the drug. Abciximab is made from the Fab fragments of an immunoglobulin that targets the glycoprotein IIb/IIIa receptor on the platelet membrane. Indications for use Abciximab is indicated for use in individuals undergoing percutaneous coronary intervention (angioplasty with or without stent placement). The use of abc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |