|
Glyoxalase
The glyoxalase system is a set of enzymes that carry out the detoxification of methylglyoxal and the other reactive aldehydes that are produced as a normal part of metabolism. This system has been studied in both bacteria and eukaryotes. This detoxification is accomplished by the sequential action of two thiol-dependent enzymes; firstly glyoxalase І, which catalyzes the isomerization of the spontaneously formed hemithioacetal adduct between glutathione and 2-oxoaldehydes (such as methylglyoxal) into S-2-hydroxyacylglutathione. Secondly, glyoxalase ІІ hydrolyses these thiolesters and in the case of methylglyoxal catabolism, produces D-lactate and GSH from S-D-lactoyl-glutathione. This system shows many of the typical features of the enzymes that dispose of endogenous toxins. Firstly, in contrast to the amazing substrate range of many of the enzymes involved in xenobiotic metabolism, it shows a narrow substrate specificity. Secondly, intracellular thiols are required as part of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactoylglutathione Lyase
The enzyme lactoylglutathione lyase (EC 4.4.1.5, also known as glyoxalase I) catalyzes the isomerization of hemithioacetal adducts, which are formed in a spontaneous reaction between a glutathionyl group and aldehydes such as methylglyoxal. :(''R'')-''S''-lactoylglutathione = glutathione + 2-oxopropanal Glyoxalase I derives its name from its catalysis of the first step in the glyoxalase system, a critical two-step detoxification system for methylglyoxal. Methylglyoxal is produced naturally as a byproduct of normal biochemistry, but is highly toxic, due to its chemical reactions with proteins, nucleic acids, and other cellular components. The second detoxification step, in which (''R'')-''S''-lactoylglutathione is split into glutathione and D-lactate, is carried out by glyoxalase II, a hydrolase. Unusually, these reactions carried out by the glyoxalase system does not oxidize glutathione, which usually acts as a redox coenzyme. Although aldose reductase can also detoxify methy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
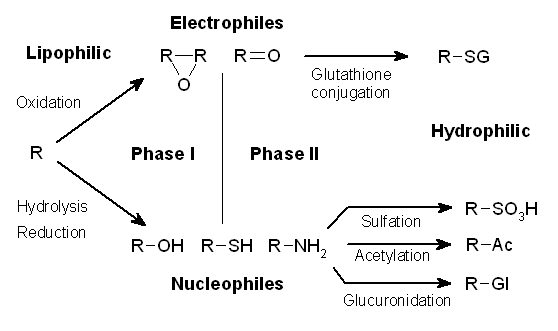
Xenobiotic Metabolism
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism (from the Greek xenos "stranger" and biotic "related to living beings") is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds (although in some cases the intermediates in xenobiotic metabolism can themselves cause toxic effects). The study of drug metabolism is called pharmacokinetics. The metabolism of pharmaceutical drugs is an important aspect of pharmacology and medicine. For example, the rate of metabolism determines the duration and intensity of a drug's pharmacologic action. Drug metabolism also affects mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylglyoxal
Methylglyoxal (MGO) is the organic compound with the formula CH3C(O)CHO. It is a reduced derivative of pyruvic acid. It is a reactive compound that is implicated in the biology of diabetes. Methylglyoxal is produced industrially by degradation of carbohydrates using overexpressed methylglyoxal synthase. Chemical structure Gaseous methylglyoxal has two carbonyl groups, an aldehyde and a ketone. In the presence of water, it exists as hydrates and oligomers. The formation of these hydrates is indicative of the high reactivity of MGO, which is relevant to its biological behavior. Biochemistry Biosynthesis and biodegradation In organisms, methylglyoxal is formed as a side-product of several metabolic pathways. Methylglyoxal mainly arises as side products of glycolysis involving glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. It is also thought to arise via the degradation of acetone and threonine. Illustrative of the myriad pathways to MGO, aristolochic acid caused 12-f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyacylglutathione Hydrolase
The enzyme hydroxyacylglutathione hydrolase (EC 3.1.2.6, systematic name = ''S''-(2-hydroxyacyl)glutathione hydrolase) catalyzes the following reaction: :''S''-(2-hydroxyacyl)glutathione + H2O = glutathione + a 2-hydroxy carboxylate This enzyme belongs to the family of hydrolases, specifically the class of thioester lyases. It is commonly known as glyoxalase II. It participates in pyruvate metabolism Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compoun .... References * EC 3.1.2 Enzymes of known structure {{3.1-enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemithioacetal
In organic chemistry, hemithioacetals (or thiohemiacetals) are organosulfur compounds with the general formula . They are the sulfur analogues of the acetals, , with an oxygen atom replaced by sulfur (as implied by the ''thio-'' prefix). Because they consist of four differing substituents on a single carbon, hemithioacetals are chiral. A related family of compounds are the dithiohemiacetals, with the formula . Although they can be important intermediates, hemithioacetals are usually not isolated, since they exist in equilibrium with thiols () and aldehydes (). Formation and structure Hemithioacetals are formed by the reaction of a thiol () and an aldehyde (): :R-CHO + R'-SH R-CH(OH)S-R' Hemithioacetals usually arise via acid catalysis. They typically are intermediates in the formation of dithioacetals (): :R-CH(OH)S-R' + R'-SH R-CH(S-R')2 + H2O Isolable hemithioacetal Hemithioacetals ordinarily readily dissociate into thiol and aldehyde, however, some have been isolated. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine. Biosynthesis and occurrence Glutathione biosynthesis involves two adenosine triphosphate-dependent steps: *First, γ-glutamylcysteine is synthesized from L- glutamate and cysteine. This conversion requires the enzyme glutamate–cysteine ligase (GCL, glutamate cysteine synthase). This reaction is the rate-limiting step in glutathione synthesis. *Second, glycine is added to the C-terminal of γ-glutamylcysteine. This condensation is catalyzed by glutathione synthetase. While all animal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminoguanidine
Pimagedine, also known as aminoguanidine, is an investigational drug for the treatment of diabetic nephropathy that is no longer under development as a drug. Pimagedine functions as an inhibitor of diamine oxidase and nitric oxide synthase. It acts to reduce levels of advanced glycation end products (AGEs) through interacting with 3-deoxyglucosone, glyoxal, methylglyoxal, and related dicarbonyls. These reactive species are converted to less reactive heterocycles by this condensation reaction. History Pimagedine was under development as a drug for kidney diseases by the pharmaceutical company Alteon (now known Synvista Therapeutics Inc.) that was founded in 1986. In 1987, Alteon acquired a license to intellectual property relating to AGE inhibition from Rockefeller University. In 1989, Alteon and Marion Merrell Dow Inc (MMD) entered into a joint development program for pimagedine. In 1992, Alteon licensed a patent from Rockefeller University relating to the use of pimagedine to i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alagebrium
Alagebrium (formerly known as ALT-711) was a drug candidate developed by Alteon, Inc. It was the first drug candidate to be clinically tested for the purpose of breaking the crosslinks caused by advanced glycation endproducts (AGEs), thereby reversing one of the main mechanisms of aging. Through this effect Alagebrium is designed to reverse the stiffening of blood vessel walls that contributes to hypertension and cardiovascular disease, as well as many other forms of degradation associated with protein crosslinking. Alagebrium has proven effective in reducing systolic blood pressure and providing therapeutic benefit for patients with diastolic heart failure. Mechanism AGEs are structures that form irreversibly when carbohydrates react non-enzymatically with proteins, lipids, or DNA. Many proteins, including structural proteins such as collagen and elastin, play an integral role in the architecture of tissues and organs and maintenance of cardiovascular elasticity and vascu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benfotiamine
Benfotiamine (rINN, or ''S''-benzoylthiamine ''O''-monophosphate) is a synthetic, fat-soluble, ''S''-acyl derivative of thiamine (vitamin B1) that is approved in some countries as a medication or dietary supplement to treat diabetic sensorimotor polyneuropathy. Benfotiamine was developed in late 1950s in Japan. Uses Benfotiamine is primarily marketed as an over-the-counter drug to treat diabetic polyneuropathy. A 2021 review described two clinical trials and concluded that more research is needed. As of 2017, benfotiamine was marketed as a pharmaceutical drug in many countries under the following brand names: Benalgis, Benfogamma, Benforce, Benfotiamina, Biotamin, Biotowa, Milgamma, and Vilotram. It was also marketed in some jurisdictions as a combination drug with cyanocobalamin as Milgamma, in combination with pyridoxine as Milgamma, in combination with metformin as Benforce-M, and with thiamine as Vitafos. Adverse effects There is little published data on adverse effects. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridoxamine
Pyridoxamine is one form of vitamin B6, vitamin B6. Chemically it is based on a pyridine ring structure, with hydroxyl, methyl, aminomethyl, and hydroxymethyl substituents. It differs from pyridoxine by the substituent at the 4-position. The hydroxyl at position 3 and aminomethyl group at position 4 of its ring endow pyridoxamine with a variety of chemical properties, including the Scavenger (chemistry), scavenging of free radical species and carbonyl species formed in sugar and lipid degradation and chelation of metal ions that catalyze Amadori rearrangement, Amadori reactions. Research Pyridoxamine can form fairly weak complexes with a number of transition metal ions, with a preference for copper, Cu2+ and Iron (element), Fe3+. The 3'-hydroxyl group of pyridoxamine allows for efficient hydroxyl radical scavenging. Pyridoxamine inhibits the Maillard reaction and can block the formation of advanced glycation endproducts, which are associated with medical complications of diabetes. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Detoxification
Detoxification or detoxication (detox for short) is the physiological or medicinal removal of toxic substances from a living organism, including the human body, which is mainly carried out by the liver. Additionally, it can refer to the period of drug withdrawal during which an organism returns to homeostasis after long-term use of an addictive substance. In medicine, detoxification can be achieved by decontamination of poison ingestion and the use of antidotes as well as techniques such as dialysis and (in a limited number of cases) chelation therapy. Many alternative medicine practitioners promote various types of detoxification such as detoxification diets. Scientists have described these as a "waste of time and money". Sense About Science, a UK-based charitable trust, determined that most such dietary "detox" claims lack any supporting evidence. The liver and kidney are naturally capable of detox, as are intracellular (specifically, inner membrane of mitochondria or in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperglycemia
Hyperglycemia is a condition in which an excessive amount of glucose circulates in the blood plasma. This is generally a blood sugar level higher than 11.1 mmol/L (200 mg/dL), but symptoms may not start to become noticeable until even higher values such as 13.9–16.7 mmol/L (~250–300 mg/dL). A subject with a consistent range between ~5.6 and ~7 mmol/L (100–126 mg/dL) ( American Diabetes Association guidelines) is considered slightly hyperglycemic, and above 7 mmol/L (126 mg/dL) is generally held to have diabetes. For diabetics, glucose levels that are considered to be too hyperglycemic can vary from person to person, mainly due to the person's renal threshold of glucose and overall glucose tolerance. On average, however, chronic levels above 10–12 mmol/L (180–216 mg/dL) can produce noticeable organ damage over time. Signs and symptoms The degree of hyperglycemia can change over time depending on the metabolic cause, for example, impaired gluco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

S.png)
