|
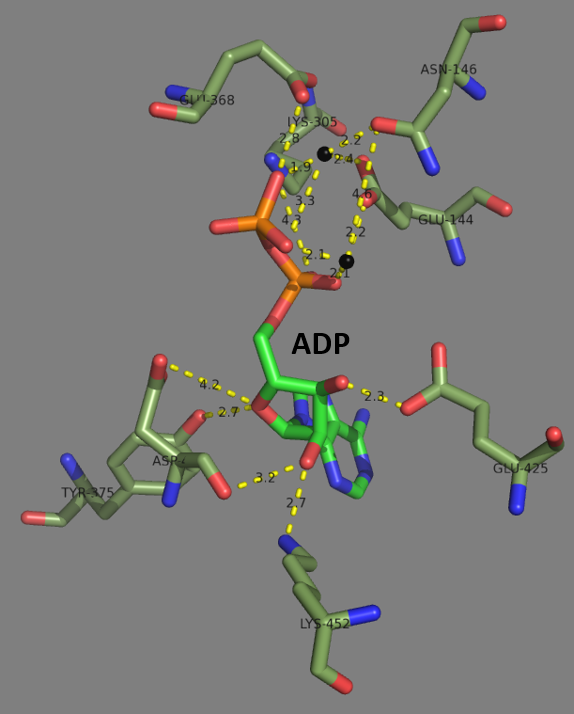
Glutamate–cysteine Ligase
Glutamate—cysteine ligase (GCL) ), previously known as γ-glutamylcysteine synthetase (GCS), is the first enzyme of the cellular glutathione (GSH) biosynthetic pathway that catalyzes the chemical reaction: L-glutamate + L-cysteine + ATP \rightleftharpoons γ-glutamyl cysteine + ADP + Pi GSH, and by extension GCL, is critical to cell survival. Nearly every eukaryotic cell, from plants to yeast to humans, expresses a form of the GCL protein for the purpose of synthesizing GSH. To further highlight the critical nature of this enzyme, genetic knockdown of GCL results in embryonic lethality. Furthermore, dysregulation of GCL enzymatic function and activity is known to be involved in the vast majority of human diseases, such as diabetes, Parkinson's disease, Alzheimer's disease, COPD, HIV/AIDS, and cancer. This typically involves impaired function leading to decreased GSH biosynthesis, reduced cellular antioxidant capacity, and the induction of oxidative stress. However, in cancer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine. Biosynthesis and occurrence Glutathione biosynthesis involves two adenosine triphosphate-dependent steps: *First, γ-glutamylcysteine is synthesized from L- glutamate and cysteine. This conversion requires the enzyme glutamate–cysteine ligase (GCL, glutamate cysteine synthase). This reaction is the rate-limiting step in glutathione synthesis. *Second, glycine is added to the C-terminal of γ-glutamylcysteine. This condensation is catalyzed by glutathione synthetase. While all animal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxicological Sciences
''Toxicological Sciences'' is a monthly peer-reviewed scientific journal which covers all aspects of research on toxicology. It is published by Oxford University Press on behalf of the Society of Toxicology. It was established in 1981 as ''Fundamental and Applied Toxicology'' and obtained its current name in 1998. The current editor-in-chief is Jeffrey M. Peters, a professor of molecular toxicology and carcinogenesis at The Pennsylvania State University, and the Managing Editor is Virginia F. Hawkins. The editorial staff also includes Associate Editors in subject areas and an editorial board of topic experts. While its ISO 4 abbreviation is ''Toxicol. Sci.'' it is commonly referred to as ''ToxSci''. Abstracting and indexing The journal is abstracted and indexed in Biological Abstracts, BIOSIS, CAB International, Chemical Abstracts Service, Current Contents, EMBASE, Health & Safety Science Abstracts, Science Citation Index, and Toxicology Abstracts. According to the ''Journal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules, such as proteins and nucleic acids. The data, typically obtained by X-ray crystallography, NMR spectroscopy, or, increasingly, cryo-electron microscopy, and submitted by biologists and biochemists from around the world, are freely accessible on the Internet via the websites of its member organisations (PDBe, PDBj, RCSB, and BMRB). The PDB is overseen by an organization called the Worldwide Protein Data Bank, wwPDB. The PDB is a key in areas of structural biology, such as structural genomics. Most major scientific journals and some funding agencies now require scientists to submit their structure data to the PDB. Many other databases use protein structures deposited in the PDB. For example, SCOP and CATH classify protein structures, while PDBsum provides a graphic overview of PDB entries using information from other sources, such as Gene ontology. History Two force ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may interact and bond in a number of ways. The interactions and bonds of side chains within a particular protein determine its tertiary structure. The protein tertiary structure is defined by its atomic coordinates. These coordinates may refer either to a protein domain or to the entire tertiary structure.Branden C. and Tooze J. "Introduction to Protein Structure" Garland Publishing, New York. 1990 and 1991. A number of tertiary structures may fold into a quaternary structure.Kyte, J. "Structure in Protein Chemistry." Garland Publishing, New York. 1995. History The science of the tertiary structure of proteins has progressed from one of hypothesis to one of detailed definition. Although Emil Fischer had suggested proteins were made of polypept ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutathione Synthetase
Glutathione synthetase (GSS) () is the second enzyme in the glutathione (GSH) biosynthesis pathway. It catalyses the condensation of gamma-glutamylcysteine and glycine, to form glutathione. Glutathione synthetase is also a potent antioxidant. It is found in many species including bacteria, yeast, mammals, and plants. In humans, defects in GSS are inherited in an autosomal recessive way and are the cause of severe metabolic acidosis, 5-oxoprolinuria, increased rate of haemolysis, and defective function of the central nervous system. Deficiencies in GSS can cause a spectrum of deleterious symptoms in plants and human beings alike. In eukaryotes, this is a homodimeric enzyme. The substrate-binding domain has a three-layer alpha/beta/alpha structure. This enzyme utilizes and stabilizes an acylphosphate intermediate to later perform a favorable nucleophilic attack of glycine. Structure Human and yeast glutathione synthetases are homodimers, meaning they are composed of two i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plastid
The plastid (Greek: πλαστός; plastós: formed, molded – plural plastids) is a membrane-bound organelle found in the Cell (biology), cells of plants, algae, and some other eukaryotic organisms. They are considered to be intracellular endosymbiotic cyanobacteria. Examples include chloroplasts (used for photosynthesis), chromoplasts (used for pigment synthesis and storage), and leucoplasts (non-pigmented plastids that can sometimes differentiate). The event which led to permanent endosymbiosis in the Archaeplastida clade (of Embryophyte, land plants, red algae, and green algae) probably occurred with a cyanobiont (a symbiotic cyanobacteria) related to the genus ''Gloeomargarita lithophora, Gloeomargarita'', around 1.5 billion years ago. A later primary endosymbiosis event occurred in photosynthetic ''Paulinella'' amoeboids about 90–140 million years ago. This plastid belongs to the "PS-clade" (of the cyanobacteria genera ''Prochlorococcus'' and ''Synechococcus''). Chloroplas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homodimer
In biochemistry, a protein dimer is a macromolecular complex formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins. A protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and heterodimers with several ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GCLM
Glutamate-cysteine ligase regulatory subunit is an enzyme that in humans is encoded by the ''GCLM'' gene. Glutamate-cysteine ligase, also known as gamma-glutamylcysteine synthetase, is the first rate limiting enzyme of glutathione synthesis. The enzyme consists of two subunits, a heavy catalytic subunit and a light regulatory subunit. Gamma glutamylcysteine synthetase deficiency has been implicated in some forms of hemolytic anemia Hemolytic anemia or haemolytic anaemia is a form of anemia due to hemolysis, the abnormal breakdown of red blood cells (RBCs), either in the blood vessels (intravascular hemolysis) or elsewhere in the human body (extravascular). This most commonly .... References Further reading * * * * * * * * * * * * * * * * * * {{gene-1-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GCLC
Glutamate—cysteine ligase catalytic subunit is an enzyme that in humans is encoded by the ''GCLC'' gene. Function Glutamate-cysteine ligase, also known as gamma-glutamylcysteine synthetase is the first rate limiting enzyme of glutathione synthesis. The enzyme consists of two subunits, a heavy catalytic subunit and a light regulatory subunit. The gene encoding the catalytic subunit encodes a protein of 367 amino acids with a calculated molecular weight of 72.773 kDa and maps to chromosome 6. The regulatory subunit is derived from a different gene located on chromosome 1p22-p21. Deficiency of gamma-glutamylcysteine synthetase in human is associated with enzymopathic hemolytic anemia. Model organisms Model organisms have been used in the study of GCLC function. A conditional knockout mouse line, called ''Gclctm1a(EUCOMM)Wtsi'' was generated as part of the International Knockout Mouse Consortium program — a high-throughput mutagenesis project to generate and distribute anim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterodimer
In biochemistry, a protein dimer is a macromolecular complex formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins. A protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and heterodimers with several typ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate Cysteine Ligase
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons. Its molecular formula is . Glutamic acid exists in three optically isomeric forms; the dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxyl groups −COOH and one amino group −. However, in the solid state and mildly acidic water solutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |