|
Glecaprevir
Glecaprevir (INN,) is a hepatitis C virus (HCV) nonstructural (NS) protein 3/ 4A protease inhibitor that was identified jointly by AbbVie and Enanta Pharmaceuticals. It is being developed as a treatment of chronic hepatitis C infection in co-formulation with an HCV NS5A inhibitor pibrentasvir. Together they demonstrated potent antiviral activity against major HCV genotypes and high barriers to resistance ''in vitro''. On 19 December 2016, AbbVie submitted a new drug application to the U.S. Food and Drug Administration The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respon ... for the glecaprevir/pibrentasvir (trade name ''Mavyret'') regimen for the treatment of all major genotypes (1–6) of chronic hepatitis C. On 3 August 2017 the FDA approved the combination for hepatitis C treatme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glecaprevir/pibrentasvir
Glecaprevir/pibrentasvir (G/P), sold under the brand names Mavyret and Maviret, is a fixed-dose combination medication used to treat hepatitis C. It contains glecaprevir and pibrentasvir. It works against all six types of hepatitis C. At twelve weeks following treatment between 81% and 100% of people have no evidence of hepatitis C. It is taken once a day by mouth with food. The most common side effects are headache, diarrhea, and tiredness. In those with a history of hepatitis B reactivation may occur. It is not recommended in people with moderate to severe liver disease. Glecaprevir works by blocking the protein NS3/ 4A protease, while pibrentasvir works by blocking NS5A. The combination was approved for medical use in the United States and Europe in 2017. It is on the World Health Organization's List of Essential Medicines. Medical uses In the United States, G/P is used to treat adults and children aged 12 years and older or weighing at least 99 pounds with chronic hepa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pibrentasvir
Pibrentasvir is an NS5A inhibitor antiviral agent. In the United States and Europe, it is approved for use with glecaprevir as the combination drug glecaprevir/pibrentasvir (trade name ''Mavyret'' in the US and ''Maviret'' in the EU) for the treatment of hepatitis C. It is sold by Abbvie AbbVie is an American publicly traded biopharmaceutical company founded in 2013. It originated as a spin-off of Abbott Laboratories. History On October 19, 2011, Abbott Laboratories announced its plan to separate into two publicly traded compani .... References {{Antiinfective-drug-stub NS5A inhibitors Fluoroarenes Benzimidazoles AbbVie brands Carbamates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AbbVie
AbbVie is an American publicly traded biopharmaceutical company founded in 2013. It originated as a spin-off of Abbott Laboratories. History On October 19, 2011, Abbott Laboratories announced its plan to separate into two publicly traded companies. The new Abbott Laboratories would specialize in diversified products including medical devices, diagnostic equipment and nutrition products, while AbbVie would operate as a research-based pharmaceutical manufacturer. The separation was effective January 1, 2013, and AbbVie was officially listed on the New York Stock Exchange (ABBV) on January 2, 2013. According to Miles White, CEO at the time, the purpose of the split was to allow markets to value the two businesses separately. Some investors were concerned that the split was done to protect the value of the device business from the loss of value facing the drug division due to the imminent expiration of patents on Humira, which accounted for about half of the drug division's revenue. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AbbVie Brands
AbbVie is an American publicly traded biopharmaceutical company founded in 2013. It originated as a spin-off of Abbott Laboratories. History On October 19, 2011, Abbott Laboratories announced its plan to separate into two publicly traded companies. The new Abbott Laboratories would specialize in diversified products including medical devices, diagnostic equipment and nutrition products, while AbbVie would operate as a research-based pharmaceutical manufacturer. The separation was effective January 1, 2013, and AbbVie was officially listed on the New York Stock Exchange (ABBV) on January 2, 2013. According to Miles White, CEO at the time, the purpose of the split was to allow markets to value the two businesses separately. Some investors were concerned that the split was done to protect the value of the device business from the loss of value facing the drug division due to the imminent expiration of patents on Humira, which accounted for about half of the drug division's revenue. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP3A
Cytochrome P450, family 3, subfamily A, also known as CYP3A, is a human gene locus. A homologous locus is found in mice. The CYP3A locus includes all the known members of the 3A subfamily of the cytochrome P450 superfamily of genes. These genes encode monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. The CYP3A cluster consists of four genes: * CYP3A4, * CYP3A5, * CYP3A7, and * CYP3A43 Cytochrome P450 3A43 is a protein that in humans is encoded by the ''CYP3A43'' gene. This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved .... The region also contains four pseudogenes: * , * , * , and * . as well as several extra exons which may or may not be included in transcripts produced from this region. Previously another CYP3A member, CYP3A3, was thought to exist; however, it is now thought that thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
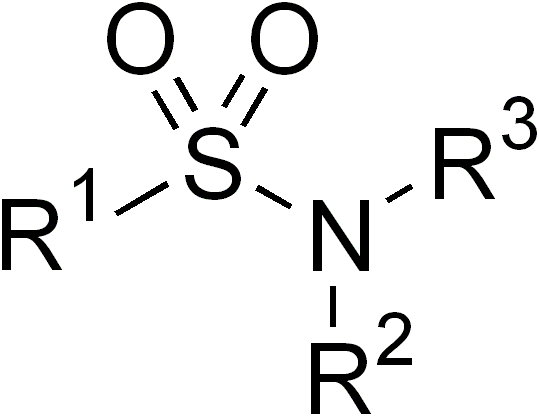
Sulfonamides
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinoxalines
A quinoxaline, also called a benzopyrazine, in organic chemistry, is a heterocyclic compound containing a ring complex made up of a benzene ring and a pyrazine ring. It is isomeric with other naphthyridines including quinazoline, phthalazine and cinnoline. It is a colorless oil that melts just above room temperature. Although quinoxaline itself is mainly of academic interest, quinoxaline derivatives are used as dyes, pharmaceuticals (such as varenicline), and antibiotics such as olaquindox, carbadox, echinomycin, levomycin and actinoleutin. Synthesis They can be formed by condensing ''ortho''-diamines with 1,2-diketones. The parent substance of the group, quinoxaline, results when glyoxal is condensed with 1,2-diaminobenzene. Substituted derivatives arise when α-ketonic acids, α-chlorketones, α-aldehyde alcohols and α-ketone alcohols are used in place of diketones. Quinoxaline and its analogues may also be formed by reduction of amino acids substituted 1,5-difluoro-2,4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrolidines
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". In addition to pyrrolidine itself, many substituted pyrrolidines are known. Production and synthesis Industrial production Pyrrolidine is prepared industrially by the reaction of 1,4-butanediol and ammonia at a temperature of 165–200 °C and a pressure of 17–21 MPa in the presence of a cobalt- and nickel oxide catalyst, which is supported on alumina. : The reaction is carried out in the liquid phase in a continuous tube- or tube bundle reactor, which is operated in the cycle gas method. The catalyst is arranged as a fixed-bed and the conversion is carried out in the downflow mode. The product is obtained after mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorides
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from Lipophobicity, oil and hydrophobe, water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/molKirsch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethers
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent C–O–C linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of oxygen in ethers, alcohols, and water is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |