|
Gallium-67
Natural gallium (31Ga) consists of a mixture of two stable isotopes: gallium-69 and gallium-71. The most commercially important radioisotopes are gallium-67 and gallium-68. Gallium-67 (half-life 3.3 days) is a gamma-emitting isotope (the gamma ray emitted immediately after electron capture) used in standard nuclear medical imaging, in procedures usually referred to as gallium scans. It is usually used as the free ion, Ga3+. It is the longest-lived radioisotope of gallium. The shorter-lived gallium-68 (half-life 68 minutes) is a positron-emitting isotope generated in very small quantities from germanium-68 in gallium-68 generators or in much greater quantities by proton bombardment of 68Zn in low-energy medical cyclotrons, for use in a small minority of diagnostic PET scans. For this use, it is usually attached as a tracer to a carrier molecule (for example the somatostatin analogue DOTATOC), which gives the resulting radiopharmaceutical a different tissue-uptake specifici ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium Scan
A gallium scan is a type of nuclear medicine test that uses either a gallium-67 (67Ga) or gallium-68 (68Ga) radiopharmaceutical to obtain images of a specific type of tissue, or disease state of tissue. Gallium salts like gallium citrate and gallium nitrate may be used. The form of salt is not important, since it is the freely dissolved gallium ion Ga3+ which is active. Both 67Ga and 68Ga salts have similar uptake mechanisms. Gallium can also be used in other forms, for example 68Ga-PSMA is used for cancer imaging. The gamma emission of gallium-67 is imaged by a gamma camera, while the positron emission of gallium-68 is imaged by positron emission tomography (PET). Gallium salts are taken up by tumors, inflammation, and both acute and chronic infection, allowing these pathological processes to be imaged. Gallium is particularly useful in imaging osteomyelitis that involves the spine, and in imaging older and chronic infections that may be the cause of a fever of unknown origin. G ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium Scan
A gallium scan is a type of nuclear medicine test that uses either a gallium-67 (67Ga) or gallium-68 (68Ga) radiopharmaceutical to obtain images of a specific type of tissue, or disease state of tissue. Gallium salts like gallium citrate and gallium nitrate may be used. The form of salt is not important, since it is the freely dissolved gallium ion Ga3+ which is active. Both 67Ga and 68Ga salts have similar uptake mechanisms. Gallium can also be used in other forms, for example 68Ga-PSMA is used for cancer imaging. The gamma emission of gallium-67 is imaged by a gamma camera, while the positron emission of gallium-68 is imaged by positron emission tomography (PET). Gallium salts are taken up by tumors, inflammation, and both acute and chronic infection, allowing these pathological processes to be imaged. Gallium is particularly useful in imaging osteomyelitis that involves the spine, and in imaging older and chronic infections that may be the cause of a fever of unknown origin. G ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Gallium
Natural gallium (31Ga) consists of a mixture of two stable isotopes: gallium-69 and gallium-71. The most commercially important radioisotopes are gallium-67 and gallium-68. Gallium-67 (half-life 3.3 days) is a gamma-emitting isotope (the gamma ray emitted immediately after electron capture) used in standard nuclear medical imaging, in procedures usually referred to as gallium scans. It is usually used as the free ion, Ga3+. It is the longest-lived radioisotope of gallium. The shorter-lived gallium-68 (half-life 68 minutes) is a positron-emitting isotope generated in very small quantities from germanium-68 in gallium-68 generators or in much greater quantities by proton bombardment of 68Zn in low-energy medical cyclotrons, for use in a small minority of diagnostic PET scans. For this use, it is usually attached as a tracer to a carrier molecule (for example the somatostatin analogue DOTATOC), which gives the resulting radiopharmaceutical a different tissue-uptake specifici ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group ( aluminium, indium, and thallium). Elemental gallium is a soft, silvery metal in standard temperature and pressure. In its liquid state, it becomes silvery white. If too much force is applied, the gallium may fracture conchoidally. Since its discovery in 1875, gallium has widely been used to make alloys with low melting points. It is also used in semiconductors, as a dopant in semiconductor substrates. The melting point of gallium is used as a temperature reference point. Gallium alloys are used in thermometers as a non-toxic and environmentally friendly alternative to mercury, and can withstand higher temperatures than mercury. An even lower melting point of , well below the freezing point of water, is claimed for the alloy galinstan ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group ( aluminium, indium, and thallium). Elemental gallium is a soft, silvery metal in standard temperature and pressure. In its liquid state, it becomes silvery white. If too much force is applied, the gallium may fracture conchoidally. Since its discovery in 1875, gallium has widely been used to make alloys with low melting points. It is also used in semiconductors, as a dopant in semiconductor substrates. The melting point of gallium is used as a temperature reference point. Gallium alloys are used in thermometers as a non-toxic and environmentally friendly alternative to mercury, and can withstand higher temperatures than mercury. An even lower melting point of , well below the freezing point of water, is claimed for the alloy galinstan ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium-68 Generator
A germanium-68/gallium-68 generator is a device used to extract the positron-emitting isotope 68Ga of gallium from a source of decaying germanium-68. The parent isotope 68Ge has a half-life of 271 days and can be easily utilized for in-hospital production of generator produced 68Ga. Its decay product gallium-68 (with a half-life of only 68 minutes, inconvenient for transport) is extracted and used for certain positron emission tomography nuclear medicine diagnostic procedures, where the radioisotope's relatively short half-life and emission of positrons for creation of 3-dimensional PET scans, are useful. Parent isotope (68Ge) source The parent isotope germanium-68 is the longest-lived (271 days) of the radioisotopes of germanium. It has been produced by several methods. In the U.S., it is primarily produced in proton accelerators: At Los Alamos National Laboratory, it may be separated out as a product of proton capture, after proton irradiation of Nb-encapsulated gallium metal. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino. : : or when written as a nuclear reaction equation, ^_e + ^_p -> ^_n + ^_ ν_e Since this single emitted neutrino carries the entire decay energy, it has this single characteristic energy. Similarly, the momentum of the neutrino emission causes the daughter atom to recoil with a single characteristic momentum. The resulting daughter nuclide, if it is in an excited state, then transitions to its ground state. Usually, a gamma ray is emitted during this transition, but nuclear de-excitation may also take place by internal conversion. Following capture of an inner electron from the atom, an outer el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pancreatic Cancer
Pancreatic cancer arises when cells in the pancreas, a glandular organ behind the stomach, begin to multiply out of control and form a mass. These cancerous cells have the ability to invade other parts of the body. A number of types of pancreatic cancer are known. The most common, pancreatic adenocarcinoma, accounts for about 90% of cases, and the term "pancreatic cancer" is sometimes used to refer only to that type. These adenocarcinomas start within the part of the pancreas that makes digestive enzymes. Several other types of cancer, which collectively represent the majority of the non-adenocarcinomas, can also arise from these cells. About 1–2% of cases of pancreatic cancer are neuroendocrine tumors, which arise from the hormone-producing cells of the pancreas. These are generally less aggressive than pancreatic adenocarcinoma. Signs and symptoms of the most-common form of pancreatic cancer may include yellow skin, abdominal or back pain, unexplained weight loss, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
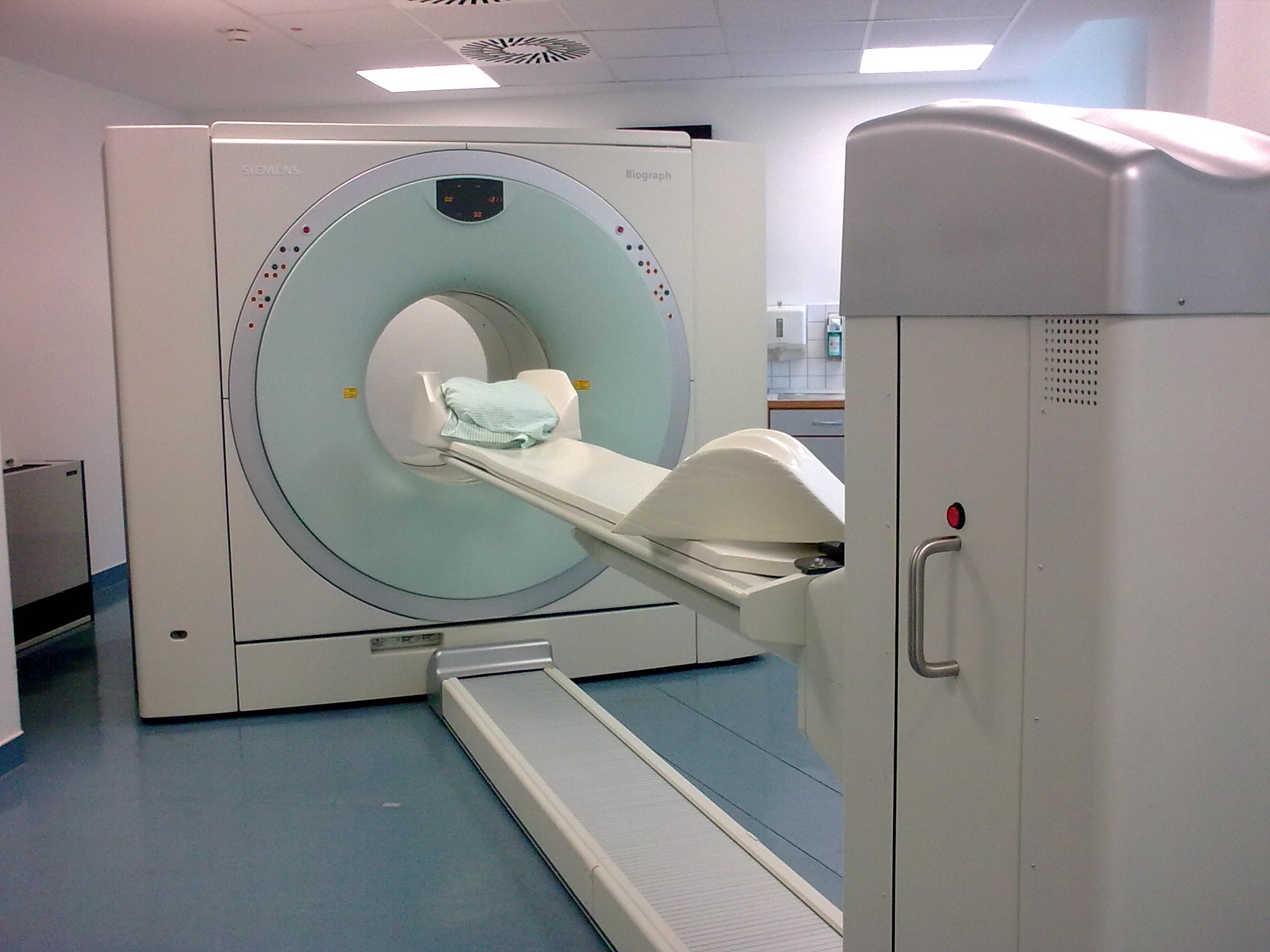
PET-CT
Positron emission tomography–computed tomography (better known as PET-CT or PET/CT) is a nuclear medicine technique which combines, in a single gantry, a positron emission tomography (PET) scanner and an x-ray computed tomography (CT) scanner, to acquire sequential images from both devices in the same session, which are combined into a single superposed ( co-registered) image. Thus, functional imaging obtained by PET, which depicts the spatial distribution of metabolic or biochemical activity in the body can be more precisely aligned or correlated with anatomic imaging obtained by CT scanning. Two- and three-dimensional image reconstruction may be rendered as a function of a common software and control system. PET-CT has revolutionized medical diagnosis in many fields, by adding precision of anatomic localization to functional imaging, which was previously lacking from pure PET imaging. For example, many diagnostic imaging procedures in oncology, surgical planning, radi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium-111
Indium-111 (111In) is a radioactive isotope of indium (In). It decays by electron capture to stable cadmium-111 with a half-life of 2.8 days. Indium-111 chloride (111InCl) solution is produced by proton irradiation of a cadmium target (112Cd(p,2n) or 111Cd(p,n)) in a cyclotron, as recommended by International Atomic Energy Agency (IAEA). The former method is more commonly used as it results in a high level of radionuclide purity. Indium-111 is commonly used in nuclear medicine diagnostic imaging by radiolabeling targeted molecules or cells. During its radioactive decay, it emits low energy gamma (γ) photons which can be imaged using planar or single-photon emission computed tomography (SPECT) gamma cameras (primary energies (ε) of 171.3 keV (91%) and 245.4 keV (94%)) Uses in nuclear medicine When formulated as an 111InCl solution, it can be used to bind antibodies, peptides, or other molecular targeted proteins or other molecules, typically using a chelate to bind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neuroendocrine Tumors
Neuroendocrine tumors (NETs) are neoplasms that arise from cells of the endocrine ( hormonal) and nervous systems. They most commonly occur in the intestine, where they are often called carcinoid tumors, but they are also found in the pancreas, lung, and the rest of the body. Although there are many kinds of NETs, they are treated as a group of tissue because the cells of these neoplasms share common features, such as looking similar, having special secretory granules, and often producing biogenic amines and polypeptide hormones. Classification WHO The World Health Organization (WHO) classification scheme places neuroendocrine tumors into three main categories, which emphasize the tumor grade rather than the anatomical origin: * well-differentiated neuroendocrine tumours, further subdivided into tumors with benign and those with uncertain behavior * well-differentiated (low grade) neuroendocrine carcinomas with low-grade malignant behavior * poorly differentiated (high grade) n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)