|
Glyoxylate And Dicarboxylate Metabolism
Glyoxylate and dicarboxylate metabolism describes a variety of reactions involving glyoxylate or dicarboxylates. Glyoxylate is the conjugate base of glyoxylic acid, and within a buffered environment of known pH such as the cell cytoplasm these terms can be used almost interchangeably, as the gain or loss of a hydrogen ion is all that distinguishes them, and this can occur in the aqueous environment at any time. Likewise dicarboxylates are the conjugate bases of dicarboxylic acids, a general class of organic compounds containing two carboxylic acid groups, such as oxalic acid or succinic acid. A compact graphical description of major biochemical reactions involved can be found at KEGG This provides information on the relevant enzymes and details the relationship with several other metabolic processes: glycine, serine, and threonine metabolism which provides hydroxypyruvate and glyoxylate, purine metabolism which provides glyoxylate, pyruvate metabolism which provides (S)-malate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
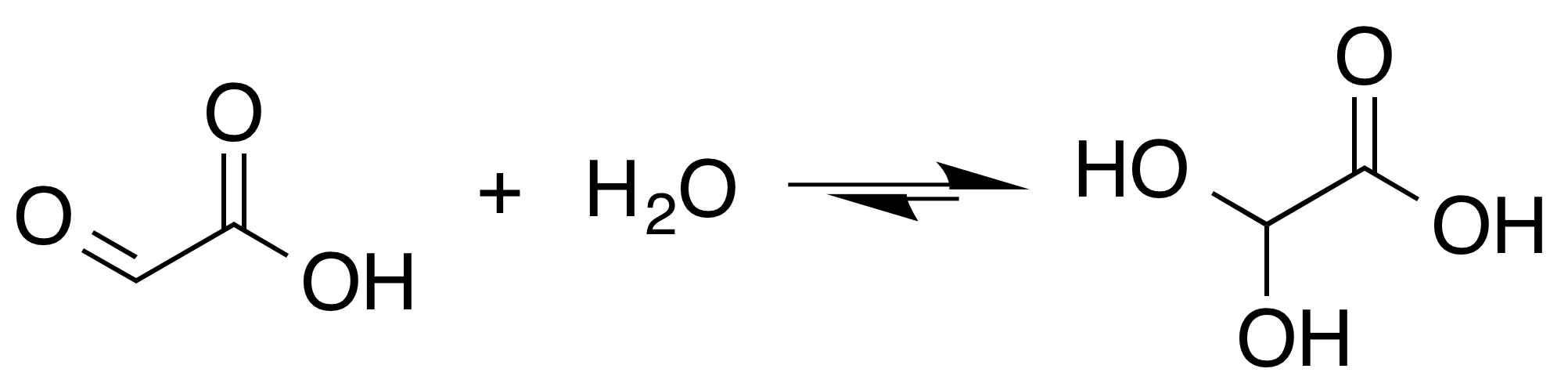
Glyoxylate
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially. Structure and nomenclature Although the structure of glyoxylic acid is described as having an aldehyde functional group, the aldehyde is only a minor component of the form most prevalent in some situations. Instead, it often exists as a hydrate or a cyclic dimer. For example, in the presence of water, the carbonyl rapidly converts to a geminal diol (described as the "monohydrate"). The equilibrium constant (''K'') is 300 for the formation of dihydroxyacetic acid at room temperature: : In solution, the monohydrate exists in equilibrium with a hemi acylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. : In isolation, the aldehyde structure ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malate
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms (L- and D-enantiomers), though only the L-isomer exists naturally. The salts and esters of malic acid are known as malates. The malate anion is an intermediate in the citric acid cycle. Etymology The word 'malic' is derived from Latin ' mālum', meaning 'apple'. The related Latin word , meaning 'apple tree', is used as the name of the genus ''Malus'', which includes all apples and crabapples; and the origin of other taxonomic classifications such as Maloideae, Malinae, and Maleae. Biochemistry L-Malic acid is the naturally occurring form, whereas a mixture of L- and D-malic acid is produced synthetically. File:L-Äpfelsäure.svg, L-Malic acid File:D-Äpfelsäure.svg, D-Malic acid Malate plays an important role in biochemistry. In the C4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl-coenzyme A
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for energy production. Coenzyme A (CoASH or CoA) consists of a β-mercaptoethylamine group linked to the vitamin pantothenic acid (B5) through an amide linkage and 3'-phosphorylated ADP. The acetyl group (indicated in blue in the structural diagram on the right) of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol). CoA is acetylated to acetyl-CoA by the breakdown of carbohydrates through glycolysis and by the breakdown of fatty acids through β-oxidation. Acetyl-CoA then enters the citric acid cycle, where the acetyl group is oxidized to carbon dioxide and wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyoxylate Cycle
The glyoxylate cycle, a variation of the tricarboxylic acid cycle, is an anabolic pathway occurring in plants, bacteria, protists, and fungi. The glyoxylate cycle centers on the conversion of acetyl-CoA to succinate for the synthesis of carbohydrates. In microorganisms, the glyoxylate cycle allows cells to use two carbons (C2 compounds), such as acetate, to satisfy cellular carbon requirements when simple sugars such as glucose or fructose are not available. The cycle is generally assumed to be absent in animals, with the exception of nematodes at the early stages of embryogenesis. In recent years, however, the detection of malate synthase (MS) and isocitrate lyase (ICL), key enzymes involved in the glyoxylate cycle, in some animal tissue has raised questions regarding the evolutionary relationship of enzymes in bacteria and animals and suggests that animals encode alternative enzymes of the cycle that differ in function from known MS and ICL in non-metazoan species. Plants as well ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolaldehyde
Glycolaldehyde is the organic compound with the formula . It is the smallest possible molecule that contains both an aldehyde group () and a hydroxyl group (). It is a highly reactive molecule that occurs both in the biosphere and in the interstellar medium. It is normally supplied as a white solid. Although it conforms to the general formula for carbohydrates, , it is not generally considered to be a saccharide. Structure Glycolaldehyde as a gas is a simple monomeric structure. As a solid and molten liquid, it exists as a dimer. Collins and George reported the equilibrium of glycolaldehyde in water by using NMR. In aqueous solution, it exists as a mixture of at least four species, which rapidly interconvert. In acidic or basic solution, the compound undergoes reversible tautomerization to form 1,2-dihydroxyethene. It is the only possible diose, a 2-carbon monosaccharide, although a diose is not strictly a saccharide. While not a true sugar, it is the simplest sugar-relate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitamin B6
Vitamin B6 is one of the B vitamins, and thus an essential nutrient. The term refers to a group of six chemically similar compounds, i.e., "vitamers", which can be interconverted in biological systems. Its active form, pyridoxal 5′-phosphate, serves as a coenzyme in more than 140 enzyme reactions in amino acid, glucose, and lipid metabolism. Plants synthesize pyridoxine as a means of protection from the ultraviolet-B radiation of sunlight and to participate in synthesis of chlorophyll. Animals cannot synthesize any of the various forms of the vitamin, and hence must obtain it via diet, either of plants, or of other animals. There is some absorption of the vitamin produced by intestinal bacteria, but this is not sufficient to meet needs. For adult humans, recommendations from various countries' food regulatory agencies are in the range of 1.0 to 2.0 milligrams (mg) per day. These same agencies also recognize ill effects from intakes that are too high, and so set safe upper l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxaloacetate
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes that occur in animals. It takes part in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, fatty acid synthesis and the citric acid cycle. Properties Oxaloacetic acid undergoes successive deprotonations to give the dianion: :HO2CC(O)CH2CO2H −O2CC(O)CH2CO2H + H+, pKa = 2.22 :−O2CC(O)CH2CO2H −O2CC(O)CH2CO2− + H+, pKa = 3.89 At high pH, the enolizable proton is ionized: :−O2CC(O)CH2CO2− −O2CC(O−)CHCO2− + H+, pKa = 13.03 The enol forms of oxaloacetic acid are particularly stable, so much so that the two tautomers have different melting points (152 °C for the ''cis'' isoform and 184 °C for the ''trans'' isoform). This reaction is catalyzed by the enzyme oxaloacetate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citrate Cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism and may have originated abiogenically. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized. The name of this metabolic pathway is derived from the citric acid (a tricarboxyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Metabolism
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems. The nitrogen cycle is of particular interest to ecologists because nitrogen availability can affect the rate of key ecosystem processes, including primary production and decomposition. Human activities such as fossil fuel combustion, use of artificial nitrogen fertilizers, and release of nitrogen in wastewater have dramatically altered the global nitr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ascorbate And Aldarate Metabolism
Vitamin C (also known as ascorbic acid and ascorbate) is a water-soluble vitamin found in citrus and other fruits and vegetables, also sold as a dietary supplement and as a topical 'serum' ingredient to treat melasma (dark pigment spots) and wrinkles on the face. It is used to prevent and treat scurvy. Vitamin C is an essential nutrient involved in the repair of tissue, the formation of collagen, and the enzymatic production of certain neurotransmitters. It is required for the functioning of several enzymes and is important for immune system function. It also functions as an antioxidant. Most animals are able to synthesize their own vitamin C. However, apes (including humans) and monkeys (but not all primates), most bats, some rodents, and certain other animals must acquire it from dietary sources. There is some evidence that regular use of supplements may reduce the duration of the common cold, but it does not appear to prevent infection. It is unclear whether supplementa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribulose
Ribulose is a ketopentose — a monosaccharide containing five carbon atoms, and including a ketone functional group. It has chemical formula . Two enantiomers are possible, -ribulose (-erythro-pentulose) and -ribulose (-erythro-pentulose). -Ribulose is the diastereomer of -xylulose. Ribulose sugars are composed in the pentose phosphate pathway from arabinose. They are important in the formation of many bioactive substances. For example, -ribulose is an intermediate in the fungal pathway for -arabitol production. Also, as the 1,5-bisphosphate, -ribulose combines with carbon dioxide at the start of the photosynthesis process in green plants (carbon dioxide trap). Ribulose has the same stereochemistry at carbons 3 and 4 as the five-carbon aldoses ribose and arabinose Arabinose is an aldopentose – a monosaccharide containing five carbon atoms, and including an aldehyde (CHO) functional group. For biosynthetic reasons, most saccharides are almost always more abundant in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |