|
Glycosylphosphatidylinositol
Glycosylphosphatidylinositol (), or glycophosphatidylinositol, or GPI in short, is a phosphoglyceride that can be attached to the C-terminus of a protein during posttranslational modification. The resulting GPI-anchored proteins play key roles in a wide variety of biological processes. GPI is composed of a phosphatidylinositol group linked through a carbohydrate-containing linker ( glucosamine and mannose glycosidically bound to the inositol residue) and via an ethanolamine phosphate (EtNP) bridge to the C-terminal amino acid of a mature protein. The two fatty acids within the hydrophobic phosphatidyl-inositol group anchor the protein to the cell membrane. Synthesis Glycosylated (GPI-anchored) proteins contain a signal sequence, thus directing them to the endoplasmic reticulum (ER). The protein is co-translationally inserted in the ER membrane via a translocon and is attached to the ER membrane by its hydrophobic C terminus; the majority of the protein extends into the ER ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glypiation
Glypiation is the addition by covalent bonding of a glycosylphosphatidylinositol (GPI) anchor and is a common post-translational modification that localizes proteins to cell membranes. This special kind of glycosylation is widely detected on surface glycoproteins in eukaryotes and some Archaea. GPI anchors consist of a phosphoethanolamine linker that binds to the C-terminus of target proteins. Glycan's core structure has a phospholipid tail that anchors the structure to the membrane. Both the lipid moiety of the tail and the sugar residues in the glycan core has considerable variation, demonstrating vast functional diversity that includes signal transduction, cell adhesion and immune recognition. GPI anchors can also be cleaved by enzymes such as phospholipase C to regulate the localization of proteins that are anchored at the plasma membrane. Mechanism Similar to the precursor glycan used for N-glycosylation, GPI anchor biosynthesis begins on the cytoplasmic leaflet of the ER an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperphosphatasia With Mental Retardation Syndrome
Hyperphosphatasia with mental retardation syndrome, HPMRS, also known as Mabry syndrome, has been described in patients recruited on four continents world-wide. Mabry syndrome was confirmed to represent an autosomal recessive syndrome characterized by severe mental retardation, considerably elevated serum levels of alkaline phosphatase, hypoplastic terminal phalanges, and distinct facial features that include: hypertelorism, a broad nasal bridge and a rectangular face. Pathogenesis While many cases of HPMRS are caused by mutations in the PIGV gene, there may be genetic heterogeneity in the spectrum of Mabry syndrome as a whole. PIGV is a member of the molecular pathway that synthesizes the glycosylphosphatidylinositol anchor. The loss in PIGV activity results in a reduced anchoring of alkaline phosphatase to the surface membrane and an elevated alkaline phosphatase activity in the serum. Diagnosis The clinical diagnosis can be established if the patient has repeatedly elevated le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placental Alkaline Phosphatase
Alkaline phosphatase, placental type also known as placental alkaline phosphatase (PLAP) is an allosteric enzyme that in humans is encoded by the ''ALPP'' gene. Gene There are at least four distinct but related alkaline phosphatases: intestinal (ALPI), placental (this enzyme), placental-like ( ALPPL2), and liver/bone/kidney ( ALPL) (tissue-nonspecific). The first three are located together on chromosome 2, whereas the tissue-nonspecific form is located on chromosome 1. The coding sequence for this form of alkaline phosphatase is unique in that the 3' untranslated region contains multiple copies of an Alu family repeat. In addition, this gene is polymorphic and three common alleles (type 1, type 2, and type 3) for this form of alkaline phosphatase have been well-characterized. Function Alkaline phosphatase, placental type is a membrane-bound glycosylated dimeric enzyme, also referred to as the heat-stable form, that is expressed primarily in the placenta, although it is clos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GPI-anchored Protein
Lipid-anchored proteins (also known as lipid-linked proteins) are proteins located on the surface of the cell membrane that are covalently attached to lipids embedded within the cell membrane. These proteins insert and assume a place in the bilayer structure of the membrane alongside the similar fatty acid tails. The lipid-anchored protein can be located on either side of the cell membrane. Thus, the lipid serves to anchor the protein to the cell membrane. They are a type of proteolipids. The lipid groups play a role in protein interaction and can contribute to the function of the protein to which it is attached. Furthermore, the lipid serves as a mediator of membrane associations or as a determinant for specific protein-protein interactions. For example, lipid groups can play an important role in increasing molecular hydrophobicity. This allows for the interaction of proteins with cellular membranes and protein domains. In a dynamic role, lipidation can sequester a protein away f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paroxysmal Nocturnal Hemoglobinuria
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired, life-threatening disease of the blood characterized by destruction of red blood cells by the complement system, a part of the body's innate immune system. This destructive process occurs due to deficiency of the red blood cell surface protein DAF, which normally inhibits such immune reactions. Since the complement cascade attacks the red blood cells within the blood vessels of the circulatory system, the red blood cell destruction (hemolysis) is considered an ''intravascular'' hemolytic anemia. Other key features of the disease, such as the high incidence of venous blood clot formation, are incompletely understood. PNH is the only hemolytic anemia caused by an ''acquired'' (rather than inherited) intrinsic defect in the cell membrane (deficiency of glycophosphatidylinositol or GPI) leading to the absence of protective exterior surface proteins that normally attach via a GPI anchor. It may develop on its own ("primary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Posttranslational Modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can extend the chemical repertoire of the 20 standard amino acids by modifying an existing functional group or introducing a new one such as phosphate. Phosphorylation is a highly effective mechanism for regulating the activity of enzymes and is the most common post-translational modification. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process called glycosyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Raft
The cell membrane, plasma membranes of cells contain combinations of glycosphingolipids, cholesterol and protein Receptor (biochemistry), receptors organised in glycolipoprotein lipid microdomains termed lipid rafts. Their existence in cellular membranes remains somewhat controversial. It has been proposed that they are specialized membrane microdomains which compartmentalize cellular processes by serving as organising centers for the assembly of signaling molecules, allowing a closer interaction of protein receptors and their effectors to promote kinetically favorable interactions necessary for the signal transduction. Lipid rafts influence membrane fluidity and membrane protein Protein targeting, trafficking, thereby regulating neurotransmission and receptor trafficking. Lipid rafts are more ordered and tightly packed than the surrounding bilayer, but float freely within the membrane bilayer. Although more common in the cell membrane, lipid rafts have also been reported in other p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ALPI
Alpi may refer to: * ALPI, an enzyme * Alpi, the Italian word for the Alps * Alpı (other) Alpı is a Turkic word that may refer to: * Alpı, Kastamonu, a village in the district of Kastamonu, Kastamonu Province, Turkey * Alpı, Ulus, a village in the district of Ulus, Bartın Province, Turkey * Alpı, Ujar, a village and municip ..., several places in Turkey and Azerbaijan * Alpi Aviation, an Italian aircraft manufacturer {{geodis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
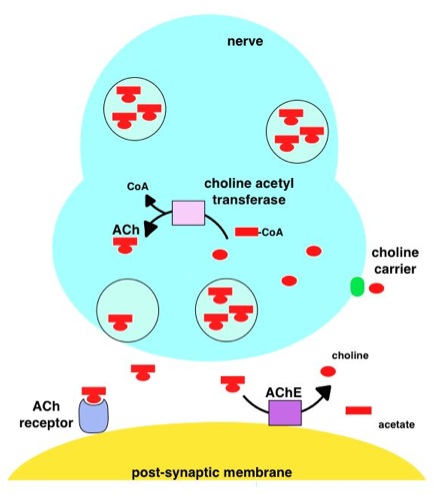
Acetylcholinesterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters: : acetylcholine + H2O = choline + acetate It is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate synaptic transmission. It belongs to the carboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides. Enzyme structure and mechanism AChE is a hydrolase that hydrolyzes choline esters. It has a very high catalytic activity—each molecule of AChE degrades about 25,000 molecules of acetylcholine (ACh) per second, approaching the limit allowed by diffusion of the substrate. The active site of AChE comprises 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
T-cell
A T cell is a type of lymphocyte. T cells are one of the important white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell receptor (TCR) on their cell surface. T cells are born from hematopoietic stem cells, found in the bone marrow. Developing T cells then migrate to the thymus gland to develop (or mature). T cells derive their name from the thymus. After migration to the thymus, the precursor cells mature into several distinct types of T cells. T cell differentiation also continues after they have left the thymus. Groups of specific, differentiated T cell subtypes have a variety of important functions in controlling and shaping the immune response. One of these functions is immune-mediated cell death, and it is carried out by two major subtypes: CD8+ "killer" and CD4+ "helper" T cells. (These are named for the presence of the cell surface proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |