|
Germanene
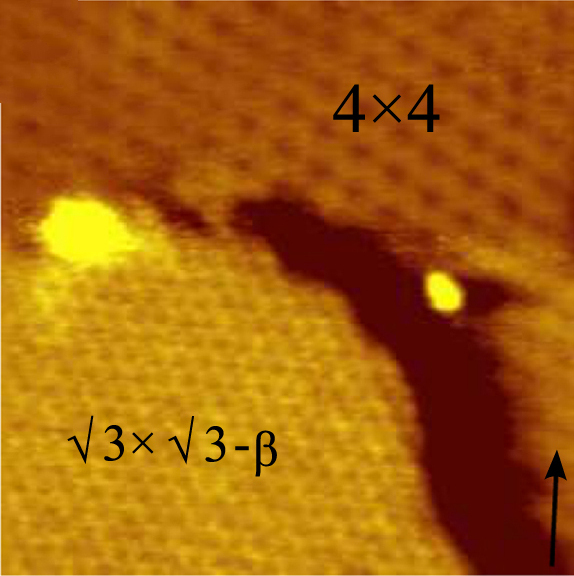
Germanene is a material made up of a single layer of germanium atoms. The material is created in a process similar to that of silicene and graphene, in which high vacuum and high temperature are used to deposit a layer of germanium atoms on a substrate. High-quality thin films of germanene have revealed unusual two-dimensional structures with novel electronic properties suitable for semiconductor device applications and materials science research. Preparation and structure In September 2014, G. Le Lay and others reported the deposition of a single atom thickness, ordered and two-dimensional multi-phase film by molecular beam epitaxy upon a gold surface in a crystal lattice with Miller indices (111). The structure was confirmed with scanning tunneling microscopy (STM) revealing a nearly flat honeycomb structure. Additional confirmation was obtained by spectroscopic measurement and density functional theory calculations. The development of high quality and nearly flat single atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germanene Microscopy
Germanene is a material made up of a single layer of germanium atoms. The material is created in a process similar to that of silicene and graphene, in which high vacuum and high temperature are used to deposit a layer of germanium atoms on a substrate. High-quality thin films of germanene have revealed unusual two-dimensional structures with novel electronic properties suitable for semiconductor device applications and materials science research. Preparation and structure In September 2014, G. Le Lay and others reported the deposition of a single atom thickness, ordered and two-dimensional multi-phase film by molecular beam epitaxy upon a gold surface in a crystal lattice with Miller indices (111). The structure was confirmed with scanning tunneling microscopy (STM) revealing a nearly flat honeycomb structure. Additional confirmation was obtained by spectroscopic measurement and density functional theory calculations. The development of high quality and nearly flat single atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Two-dimensional Nanomaterials
In materials science, the term single-layer materials or 2D materials refers to crystalline solids consisting of a single layer of atoms. These materials are promising for some applications but remain the focus of research. Single-layer materials derived from single elements generally carry the -ene suffix in their names, e.g. graphene. Single-layer materials that are compounds of two or more elements have -ane or -ide suffixes. 2D materials can generally be categorized as either 2D allotropes of various elements or as compounds (consisting of two or more covalently bonding elements). It is predicted that there are hundreds of stable single-layer materials. The atomic structure and calculated basic properties of these and many other potentially synthesisable single-layer materials, can be found in computational databases. 2D materials can be produced using mainly two approaches: top-down exfoliation and bottom-up synthesis. The exfoliation methods include sonication, mechanical, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicene
Silicene is a two-dimensional allotrope of silicon, with a hexagonal honeycomb structure similar to that of graphene. Contrary to graphene, silicene is not flat, but has a periodically buckled topology; the coupling between layers in silicene is much stronger than in multilayered graphene; and the oxidized form of silicene, 2D silica, has a very different chemical structure from graphene oxide. History Although theorists had speculated about the existence and possible properties of free-standing silicene, researchers first observed silicon structures that were suggestive of silicene in 2010. Using a scanning tunneling microscope they studied self-assembled silicene nanoribbons and silicene sheets deposited onto a silver crystal, Ag(110) and Ag(111), with atomic resolution. The images revealed hexagons in a honeycomb structure similar to that of graphene, which, however, were shown to originate from the silver surface mimicking the hexagons. Density functional theory (DFT) cal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure. "Carbon nanostructures for electromagnetic shielding applications", Mohammed Arif Poothanari, Sabu Thomas, et al., ''Industrial Applications of Nanomaterials'', 2019. "Carbon nanostructures include various low-dimensional allotropes of carbon including carbon black (CB), carbon fiber, carbon nanotubes (CNTs), fullerene, and graphene." The name is derived from "graphite" and the suffix -ene, reflecting the fact that the allotrope of carbon contains numerous double bonds. Each atom in a graphene sheet is connecte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropes
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the atoms of the element are bonded together in a different manner. For example, the allotropes of carbon include diamond (the carbon atoms are bonded together to form a cubic lattice of tetrahedra), graphite (the carbon atoms are bonded together in sheets of a hexagonal lattice), graphene (single sheets of graphite), and fullerenes (the carbon atoms are bonded together in spherical, tubular, or ellipsoidal formations). The term ''allotropy'' is used for elements only, not for compounds. The more general term, used for any compound, is polymorphism, although its use is usually restricted to solid materials such as crystals. Allotropy refers only to different forms of an element within the same physical phase (the state of matter, such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors silicon and tin. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature. Because it seldom appears in high concentration, germanium was discovered comparatively late in the discovery of the elements. Germanium ranks near fiftieth in relative abundance of the elements in the Earth's crust. In 1869, Dmitri Mendeleev predicted its existence and some of its properties from its position on his periodic table, and called the element ekasilicon. In 1886, Clemens Winkler at Freiberg University found the new element, along with silver and sulfur, in the mineral argyrodite. Winkler named the element after his country, Germany. Germanium is mined primarily from sphalerite (the primary ore of zinc), though germanium is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors silicon and tin. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature. Because it seldom appears in high concentration, germanium was discovered comparatively late in the discovery of the elements. Germanium ranks near fiftieth in relative abundance of the elements in the Earth's crust. In 1869, Dmitri Mendeleev predicted its existence and some of its properties from its position on his periodic table, and called the element ekasilicon. In 1886, Clemens Winkler at Freiberg University found the new element, along with silver and sulfur, in the mineral argyrodite. Winkler named the element after his country, Germany. Germanium is mined primarily from sphalerite (the primary ore of zinc), though germanium is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Field-effect Transistor
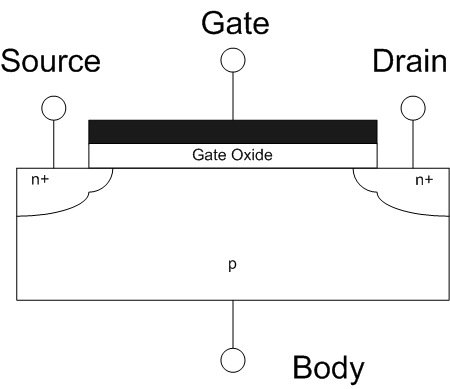
The field-effect transistor (FET) is a type of transistor that uses an electric field to control the flow of current in a semiconductor. FETs (JFETs or MOSFETs) are devices with three terminals: ''source'', ''gate'', and ''drain''. FETs control the flow of current by the application of a voltage to the gate, which in turn alters the conductivity between the drain and source. FETs are also known as unipolar transistors since they involve single-carrier-type operation. That is, FETs use either electrons (n-channel) or holes (p-channel) as charge carriers in their operation, but not both. Many different types of field effect transistors exist. Field effect transistors generally display very high input impedance at low frequencies. The most widely used field-effect transistor is the MOSFET (metal-oxide-semiconductor field-effect transistor). History The concept of a field-effect transistor (FET) was first patented by Austro-Hungarian physicist Julius Edgar Lilienfeld in 192 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group IV Semiconductors
A group is a number of persons or things that are located, gathered, or classed together. Groups of people * Cultural group, a group whose members share the same cultural identity * Ethnic group, a group whose members share the same ethnic identity * Religious group (other), a group whose members share the same religious identity * Social group, a group whose members share the same social identity * Tribal group, a group whose members share the same tribal identity * Organization, an entity that has a collective goal and is linked to an external environment * Peer group, an entity of three or more people with similar age, ability, experience, and interest Social science * In-group and out-group * Primary, secondary, and reference groups * Social group * Collectives Science and technology Mathematics * Group (mathematics), a set together with a binary operation satisfying certain algebraic conditions Chemistry * Functional group, a group of atoms which provide s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plumbene
Plumbene is a material made up of a single layer of lead atoms. The material is created in a process similar to that of graphene, silicene, germanene, and stanene, in which high vacuum and high temperature are used to deposit a layer of lead atoms on a substrate. High-quality thin films of plumbene have revealed two-dimensional honeycomb structures. First researched by Indian scientists, further investigations are being done around the world. Preparation and structure In April 2019, J. Yuhara and others reported the deposition of a single atom thickness by molecular beam epitaxy with a segregation method upon a palladium surface in a crystal lattice with Miller indices (111). The structure was confirmed with scanning tunneling microscopy (STM) revealing a nearly flat honeycomb structure. There is no evidence of any three-dimensional islands, but one notices a unique nanostructured tessellation all over the terraces looking like a space-filling polyhedral foam reduced to dimension 2. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stanene
Stanene is a topological insulator, theoretically predicted by Prof. Shoucheng Zhang's group at Stanford, which may display dissipationless currents at its edges near room temperature. It is composed of tin atoms arranged in a single layer, in a manner similar to graphene. Stanene got its name by combining ''stannum'' (the List of chemical element name etymologies, Latin name for tin) with the suffix ''-ene'' used by graphene. Research is ongoing in Germany and China, as well as at laboratories at Stanford and UCLA. The addition of fluorine atoms to the tin lattice could extend the critical temperature up to 100 °C. This would make it practical for use in integrated circuits to make smaller, faster and more energy efficient computers. See also *Graphene *Silicene Silicene is a two-dimensional allotrope of silicon, with a hexagonal honeycomb structure similar to that of graphene. Contrary to graphene, silicene is not flat, but has a periodically buckled topology; the cou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band Gap
In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in electron volts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote a valence electron bound to an atom to become a conduction electron, which is free to move within the crystal lattice and serve as a charge carrier to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move within the solid because there are no available states. If the electrons are not free to move within the crystal lattice, then there is no generated current due to no net charge carrier mobility. However, if some electrons transfer from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |