|
Gelatinases
Gelatinases are enzymes capable of degrading gelatin. Gelatinases are expressed in several bacteria including ''Pseudomonas aeruginosa'' and ''Serratia marcescens''. In humans, the gelatinases are matrix metalloproteinases MMP2 and MMP9. References EC 3.4.24 {{enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Matrix Metalloproteinases
Matrix metalloproteinases (MMPs), also known as matrix metallopeptidases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other family members are adamalysins, serralysins, and astacins. The MMPs belong to a larger family of proteases known as the metzincin superfamily. Collectively, these enzymes are capable of degrading all kinds of extracellular matrix proteins, but also can process a number of Biological activity, bioactive molecules. They are known to be involved in the cleavage of cell surface Receptor (biochemistry), receptors, the release of apoptosis, apoptotic ligands (such as the FAS ligand), and chemokine/cytokine inactivation. MMPs are also thought to play a major role in cell behaviors such as cell proliferation, cell migration, migration (cell adhesion, adhesion/dispersion), Cellular differentiation, differentiation, angiogenesis, apoptosis, and Immune system, host defense. They were first described in vertebrates (19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MMP2
72 kDa type IV collagenase also known as matrix metalloproteinase-2 (MMP-2) and gelatinase A is an enzyme that in humans is encoded by the ''MMP2'' gene. The ''MMP2'' gene is located on chromosome 16 at position 12.2. Function Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix (ECM) in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis. Most MMP's are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. This gene encodes an enzyme which degrades type IV collagen, the major structural component of basement membranes. The enzyme plays a role in endometrial menstrual breakdown, regulation of vascularization and the inflammatory response. Activation Activation of MMP-2 requires proteolytic processing. A complex of membrane type 1 MMP (MT1-MMP/MMP14) and tissue inhibit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzymes
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are Ribozyme, catalytic RNA molecules, called ribozymes. Enzymes' Chemical specificity, specific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gelatin
Gelatin or gelatine (from la, gelatus meaning "stiff" or "frozen") is a translucent, colorless, flavorless food ingredient, commonly derived from collagen taken from animal body parts. It is brittle when dry and rubbery when moist. It may also be referred to as hydrolyzed collagen, collagen hydrolysate, gelatine hydrolysate, hydrolyzed gelatine, and collagen peptides after it has undergone hydrolysis. It is commonly used as a gelling agent in food, beverages, medications, drug or vitamin capsules, photographic films, papers, and cosmetics. Substances containing gelatin or functioning in a similar way are called gelatinous substances. Gelatin is an irreversibly hydrolyzed form of collagen, wherein the hydrolysis reduces protein fibrils into smaller peptides; depending on the physical and chemical methods of denaturation, the molecular weight of the peptides falls within a broad range. Gelatin is present in gelatin desserts, most gummy candy and marshmallows, ice creams, dips ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
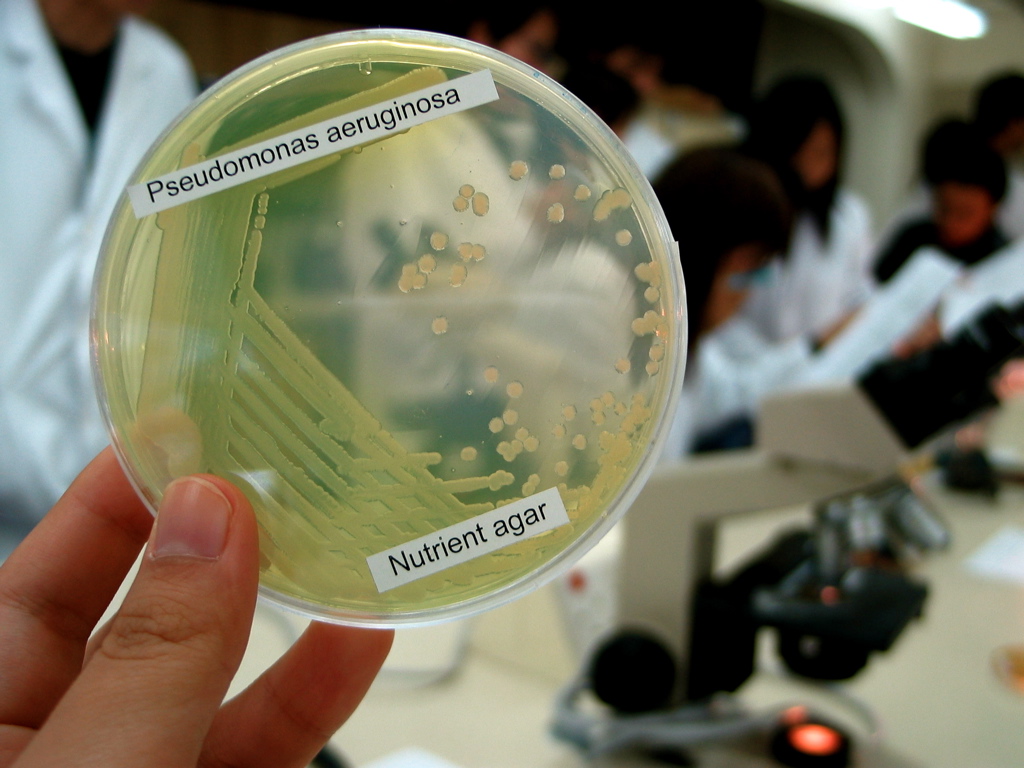
Pseudomonas Aeruginosa
''Pseudomonas aeruginosa'' is a common encapsulated, gram-negative, aerobic–facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, ''P. aeruginosa'' is a multidrug resistant pathogen recognized for its ubiquity, its intrinsically advanced antibiotic resistance mechanisms, and its association with serious illnesses – hospital-acquired infections such as ventilator-associated pneumonia and various sepsis syndromes. The organism is considered opportunistic insofar as serious infection often occurs during existing diseases or conditions – most notably cystic fibrosis and traumatic burns. It generally affects the immunocompromised but can also infect the immunocompetent as in hot tub folliculitis. Treatment of ''P. aeruginosa'' infections can be difficult due to its natural resistance to antibiotics. When more advanced antibiotic drug regimens are needed adverse effects may re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serratia Marcescens
''Serratia marcescens'' () is a species of rod-shaped, Gram-negative bacteria in the family Yersiniaceae. It is a facultative anaerobe and an opportunistic pathogen in humans. It was discovered in 1819 by Bartolomeo Bizio in Padua, Italy.Serratia marcescens. (2011, April). Retrieved from https://microbewiki.kenyon.edu/index.php/Serratia_marcescens ''S. marcescens'' is commonly involved in hospital-acquired infections (HAIs), also called nosocomial infections, particularly catheter-associated bacteremia, urinary tract infections, and wound infections, and is responsible for 1.4% of HAI cases in the United States. It is commonly found in the respiratory and urinary tracts of hospitalized adults and in the gastrointestinal systems of children. Due to its abundant presence in the environment, and its preference for damp conditions, ''S. marcescens'' is commonly found growing in bathrooms (especially on tile grout, shower corners, toilet water lines, and basins), where it manife ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MMP9
Matrix metallopeptidase 9 (MMP-9), also known as 92 kDa type IV collagenase, 92 kDa gelatinase or gelatinase B (GELB), is a matrixin, a class of enzymes that belong to the zinc-metalloproteinases family involved in the degradation of the extracellular matrix. In humans the ''MMP9'' gene encodes for a signal peptide, a propeptide, a catalytic domain with inserted three repeats of fibronectin type II domain followed by a C-terminal hemopexin-like domain. Function Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, angiogenesis, bone development, wound healing, cell migration, learning and memory, as well as in pathological processes, such as arthritis, intracerebral hemorrhage, and metastasis. Most MMPs are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. The enzyme encoded by this gene degrades typ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |