|
Fuel-cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen (usually from air) to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from substances that are already present in the battery. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied. The first fuel cells were invented by Sir William Grove in 1838. The first commercial use of fuel cells came more than a century later following the invention of the hydrogen–oxygen fuel cell by Francis Thomas Bacon in 1932. The alkaline fuel cell, also known as the Bacon fuel cell after its inventor, has been used in NASA space programs since the mid-1960s to generate power for satellites and space capsules. Since then, fuel cells have bee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fuel Cell Vehicle
A fuel cell vehicle (FCV) or fuel cell electric vehicle (FCEV) is an electric vehicle that uses a fuel cell, sometimes in combination with a small battery or supercapacitor, to power its onboard electric motor. Fuel cells in vehicles generate electricity generally using oxygen from the air and compressed hydrogen. Most fuel cell vehicles are classified as zero-emissions vehicles that emit only water and heat. As compared with internal combustion vehicles, hydrogen vehicles centralize pollutants at the site of the hydrogen production, where hydrogen is typically derived from reformed natural gas. Transporting and storing hydrogen may also create pollutants. Fuel cells have been used in various kinds of vehicles including forklifts, especially in indoor applications where their clean emissions are important to air quality, and in space applications. The first commercially produced hydrogen fuel cell automobile, the Hyundai ix35 FCEV, was introduced in 2013, the Toyota Mirai followed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton-exchange Membrane Fuel Cell
Proton-exchange membrane fuel cells (PEMFC), also known as proton-exchange membrane, polymer electrolyte membrane (PEM) fuel cells, are a type of fuel cell being developed mainly for transport applications, as well as for stationary fuel-cell applications and Fuel cell#Portable power systems, portable fuel-cell applications. Their distinguishing features include lower temperature/pressure ranges (50 to 100 °C) and a special proton-conducting polymer electrolyte membrane. PEMFCs generate electricity and operate on the opposite principle to PEM electrolysis, which consumes electricity. They are a leading candidate to replace the aging Alkaline fuel cell, alkaline fuel-cell technology, which was used in the Space Shuttle. Science PEMFCs are built out of Membrane electrode assembly, membrane electrode assemblies (MEA) which include the electrodes, electrolyte, catalyst, and gas diffusion layers. An ink of catalyst, carbon, and electrode are sprayed or painted onto the solid ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoric Acid Fuel Cell
Phosphoric acid fuel cells (PAFC) are a type of fuel cell that uses liquid phosphoric acid as an electrolyte. They were the first fuel cells to be commercialized. Developed in the mid-1960s and field-tested since the 1970s, they have improved significantly in stability, performance, and cost. Such characteristics have made the PAFC a good candidate for early stationary applications. Design Electrolyte is highly concentrated or pure liquid phosphoric acid (H3PO4) saturated in a silicon carbide (SiC) matrix. Operating range is about 150 to 210 °C. The electrodes are made of carbon paper coated with a finely dispersed platinum catalyst. Electrode reactions Anode reaction: 2H2(g) → 4H+ + 4e‾ Cathode reaction: O2(g) + 4H+ + 4e‾ → 2H2O Overall cell reaction: 2 H2 + O2 → 2H2O Advantages and disadvantages At an operating range of 150 to 200 °C, the expelled water can be converted to steam for air and water heating (combined heat and power). This potentially allow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid Oxide Fuel Cell
A solid oxide fuel cell (or SOFC) is an electrochemical conversion device that produces electricity directly from oxidizing a fuel. Fuel cells are characterized by their electrolyte material; the SOFC has a solid oxide or ceramic electrolyte. Advantages of this class of fuel cells include high combined heat and power efficiency, long-term stability, fuel flexibility, low emissions, and relatively low cost. The largest disadvantage is the high operating temperature which results in longer start-up times and mechanical and chemical compatibility issues. Introduction Solid oxide fuel cells are a class of fuel cells characterized by the use of a solid oxide material as the electrolyte. SOFCs use a solid oxide electrolyte to conduct negative oxygen ions from the cathode to the anode. The electrochemical oxidation of the hydrogen, carbon monoxide or other organic intermediates by oxygen ions thus occurs on the anode side. More recently, proton-conducting SOFCs (PC-SOFC) are being deve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allis-Chalmers
Allis-Chalmers was a U.S. manufacturer of machinery for various industries. Its business lines included agricultural equipment, construction equipment, power generation and power transmission equipment, and machinery for use in industrial settings such as factories, flour mills, sawmills, textile mills, steel mills, refineries, mines, and ore mills. The first Allis-Chalmers Company was formed in 1901 as an amalgamation of the Edward P. Allis Company (steam engines and mill equipment), Fraser & Chalmers (mining and ore milling equipment), the Gates Iron Works (rock and cement milling equipment), and the industrial business line of the Dickson Manufacturing Company (engines and compressors). It was reorganized in 1912 as the Allis-Chalmers Manufacturing Company. During the next 70 years its industrial machinery filled countless mills, mines, and factories around the world, and its brand gained fame among consumers mostly from its farm equipment business's orange tractors and s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Cell
An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. The electrochemical cells which generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells. A common example of a galvanic cell is a standard 1.5 volt cell meant for consumer use. A ''battery'' consists of one or more cells, connected in parallel, series or series-and-parallel pattern. Electrolytic cell An electrolytic cell is an electrochemical cell that drives a non-spontaneous redox reaction through the application of electrical energy. They are often used to decompose chemical compounds, in a process called electrolysis—the Greek word lysis means ''to break up''. Important examples of electrolysis are the decomposition of water into hydrogen and oxygen, and bauxite into aluminium and other chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
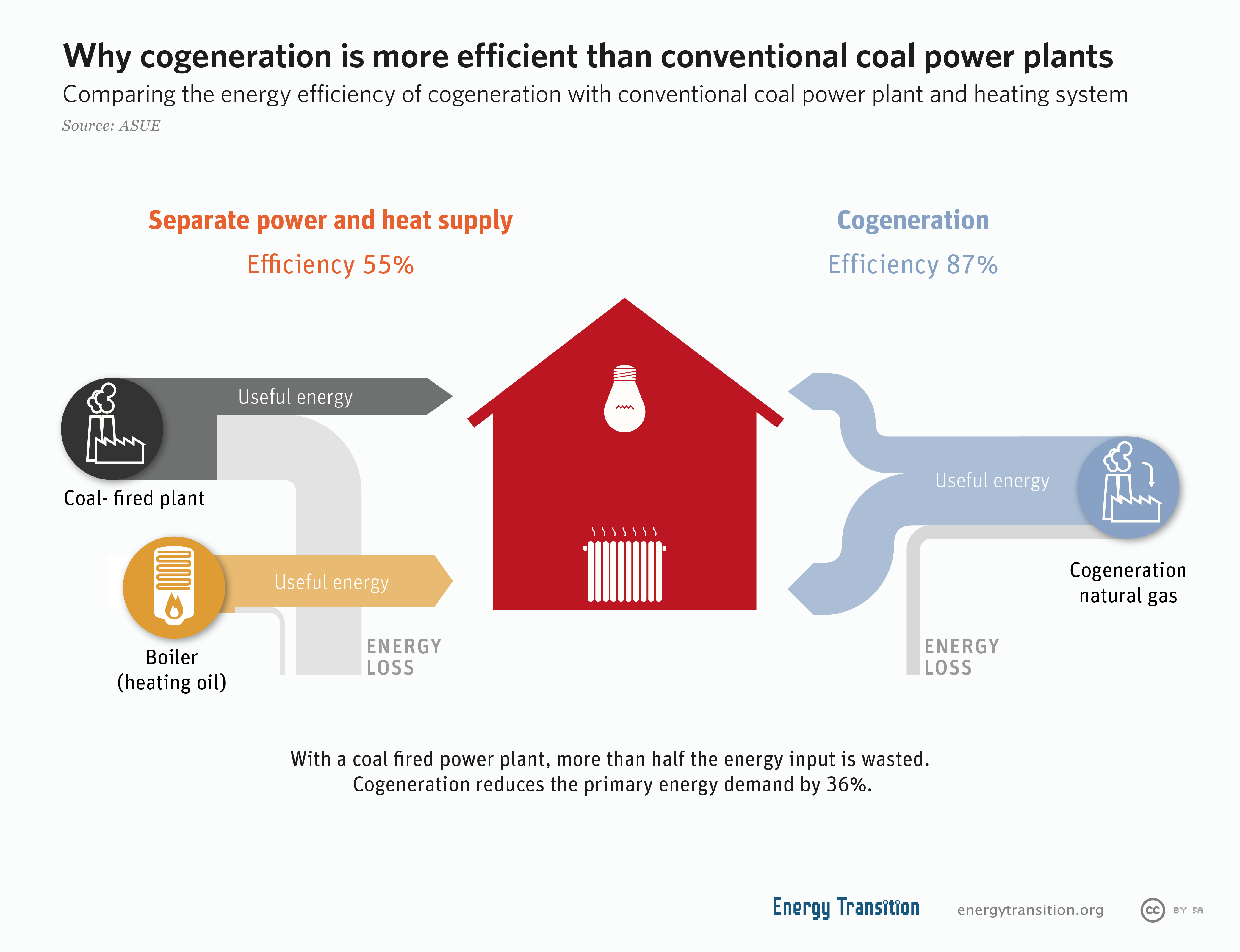
Cogeneration
Cogeneration or combined heat and power (CHP) is the use of a heat engine or power station to generate electricity and useful heat at the same time. Cogeneration is a more efficient use of fuel or heat, because otherwise- wasted heat from electricity generation is put to some productive use. Combined heat and power (CHP) plants recover otherwise wasted thermal energy for heating. This is also called combined heat and power district heating. Small CHP plants are an example of decentralized energy. By-product heat at moderate temperatures (100–180 °C, 212–356 °F) can also be used in absorption refrigerators for cooling. The supply of high-temperature heat first drives a gas or steam turbine-powered generator. The resulting low-temperature waste heat is then used for water or space heating. At smaller scales (typically below 1 MW), a gas engine or diesel engine may be used. Cogeneration is also common with geothermal power plants as they often produce relatively lo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Fuel Cell
The alkaline fuel cell (AFC), also known as the Bacon fuel cell after its British inventor, Francis Thomas Bacon, is one of the most developed fuel cell technologies. Alkaline fuel cells consume hydrogen and pure oxygen, to produce potable water, heat, and electricity. They are among the most efficient fuel cells, having the potential to reach 70%. NASA has used alkaline fuel cells since the mid-1960s, in the Apollo-series missions and on the Space Shuttle. Half Reactions The fuel cell produces power through a redox reaction between hydrogen and oxygen. At the anode, hydrogen is oxidized according to the reaction: \mathrm_2 + \mathrm^- \longrightarrow \mathrm_2\mathrm + \mathrm^- producing water and releasing electrons. The electrons flow through an external circuit and return to the cathode, reducing oxygen in the reaction: \mathrm_2 + \mathrm_2\mathrm + \mathrm^- \longrightarrow \mathrm^- producing hydroxide ions. The net reaction consumes one oxygen molecule and two h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flow Battery
A flow battery, or redox flow battery (after reduction–oxidation), is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane. Ion transfer inside the cell (accompanied by flow of electric current through an external circuit) occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts. The energy capacity is a function of the electrolyte volume and the power is a function of the surface area of the electrodes. A flow battery may be used like a fuel cell (where the spent fuel is extracted and new fuel is added to the system) or like a rechargeable battery (where an electric power source drives regeneration of the fuel). While flow batteries have certain technical advantages over conventional rechargeabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Dioxide
Nitrogen dioxide is a chemical compound with the formula . It is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the production of fertilizers. At higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantities. Nitrogen dioxide is a paramagnetic, bent molecule with C2v point group symmetry. It is included in the NOx family of atmospheric pollutants. Properties Nitrogen dioxide is a reddish-brown gas with a pungent, acrid odor above , becomes a yellowish-brown liquid below , and converts to the colorless dinitrogen tetroxide () below . The bond length between the nitrogen atom and the oxygen atom is 119.7 pm. This bond length is consistent with a bond order between one and two. Unlike ozone, O3, the ground electronic state of nitrogen dioxide is a doublet state, since nitrogen has one unpaired electron, which decreases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fuel Cell NASA P48600ac
A fuel is any material that can be made to react with other substances so that it releases energy as thermal energy or to be used for work (physics), work. The concept was originally applied solely to those materials capable of releasing chemical energy but has since also been applied to other sources of heat energy, such as Nuclear power, nuclear energy (via nuclear fission and nuclear fusion). The heat energy released by reactions of fuels can be converted into mechanical energy via a heat engine. Other times, the heat itself is valued for warmth, cooking, or industrial processes, as well as the illumination that accompanies combustion. Fuels are also used in the Cell (biology), cells of organisms in a process known as cellular respiration, where organic molecules are oxidized to release usable energy. Hydrocarbons and related organic molecules are by far the most common source of fuel used by humans, but other substances, including radioactive metals, are also utilized. Fu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1839 William Grove Fuel Cell
Events January–March * January 2 – The first photograph of the Moon is taken, by French photographer Louis Daguerre. * January 6 – Night of the Big Wind: Ireland is struck by the most damaging cyclone in 300 years. * January 9 – The French Academy of Sciences announces the daguerreotype photography process. * January 19 – British forces capture Aden. * January 20 – Battle of Yungay: Chile defeats the Peru–Bolivian Confederation, leading to the restoration of an independent Peru. * January – The first parallax measurement of the distance to Alpha Centauri is published by Thomas Henderson. * February 11 – The University of Missouri is established, becoming the first public university west of the Mississippi River. * February 24 – William Otis receives a patent for the steam shovel. * March 5 – Longwood University is founded in Farmville, Virginia. * March 7 – Baltimore City College, the third public high school in the United States, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |