|
Free Water Clearance
In the physiology of the kidney, free water clearance (CH2O) is the volume of blood plasma that is cleared of solute-free water per unit time. An example of its use is in the determination of an individual's state of hydration. Conceptually, free water clearance should be thought of relative to the production of isoosmotic urine, which would be equal to the osmolarity of the plasma. If an individual is producing urine more dilute than the plasma, there is a positive value for free water clearance, meaning pure water is lost in the urine in addition to a theoretical isoosmotic filtrate. If the urine is more concentrated than the plasma, then free water is being extracted from the urine, giving a negative value for free water clearance. A negative value is typical for free water clearance, as the kidney usually produces concentrated urine except in the cases of volume overload by the individual. Overview At its simplest, the kidney produces urine composed of solute and pure (solute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Renal Physiology
Renal physiology (Latin ''rēnēs'', "kidneys") is the study of the physiology of the kidney. This encompasses all functions of the kidney, including maintenance of acid-base balance; regulation of fluid balance; regulation of sodium, potassium, and other electrolytes; clearance of toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D. Much of renal physiology is studied at the level of the nephron, the smallest functional unit of the kidney. Each nephron begins with a filtration component that filters the blood entering the kidney. This filtrate then flows along the length of the nephron, which is a tubular structure lined by a single layer of specialized cells and surrounded by capillaries. The major functions of these lining cells are the reabsorption of water and small molecules from the filtrate into the blood, and the secretion of wastes f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kidney
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; blood exits into the paired renal veins. Each kidney is attached to a ureter, a tube that carries excreted urine to the bladder. The kidney participates in the control of the volume of various body fluids, fluid osmolality, acid–base balance, various electrolyte concentrations, and removal of toxins. Filtration occurs in the glomerulus: one-fifth of the blood volume that enters the kidneys is filtered. Examples of substances reabsorbed are solute-free water, sodium, bicarbonate, glucose, and amino acids. Examples of substances secreted are hydrogen, ammonium, potassium and uric acid. The nephron is the structural and functional unit of the kidney. Each adult human kidney contains around 1 million nephrons, while a mouse kidney contains on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Plasma
Blood plasma is a light amber-colored liquid component of blood in which blood cells are absent, but contains proteins and other constituents of whole blood in suspension. It makes up about 55% of the body's total blood volume. It is the intravascular part of extracellular fluid (all body fluid outside cells). It is mostly water (up to 95% by volume), and contains important dissolved proteins (6–8%; e.g., serum albumins, globulins, and fibrinogen), glucose, clotting factors, electrolytes (, , , , , etc.), hormones, carbon dioxide (plasma being the main medium for excretory product transportation), and oxygen. It plays a vital role in an intravascular osmotic effect that keeps electrolyte concentration balanced and protects the body from infection and other blood-related disorders. Blood plasma is separated from the blood by spinning a vessel of fresh blood containing an anticoagulant in a centrifuge until the blood cells fall to the bottom of the tube. The blood plasma is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Renal Clearance
In pharmacology, clearance is a pharmacokinetic measurement of the volume of plasma from which a substance is completely removed per unit time. Usually, clearance is measured in L/h or mL/min. The quantity reflects the rate of drug elimination divided by plasma concentration. Excretion, on the other hand, is a measurement of the amount of a substance removed from the body per unit time (e.g., mg/min, μg/min, etc.). While clearance and excretion of a substance are related, they are not the same thing. The concept of clearance was described by Thomas Addis, a graduate of the University of Edinburgh Medical School. Substances in the body can be cleared by various organs, including the kidneys, liver, lungs, etc. Thus, total body clearance is equal to the sum clearance of the substance by each organ (e.g., renal clearance + hepatic clearance + lung clearance = total body clearance). For many drugs, however, clearance is solely a function of renal excretion. In these cases, clearance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solute
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. These surrounded solute particles then move away from the solid solute and out into the solution. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food, energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. "Water" is also the name of the liquid state of H2O at standard temperature and pressure. A number of natural states of water exist. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. Water co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmolarity
Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution (osmol/L or Osm/L). The osmolarity of a solution is usually expressed as Osm/L (pronounced "osmolar"), in the same way that the molarity of a solution is expressed as "M" (pronounced "molar"). Whereas molarity measures the number of moles of solute per unit volume of solution, osmolarity measures the number of ''osmoles of solute particles'' per unit volume of solution. This value allows the measurement of the osmotic pressure of a solution and the determination of how the solvent will diffuse across a semipermeable membrane (osmosis) separating two solutions of different osmotic concentration. Unit The unit of osmotic concentration is the osmole. This is a non- SI unit of measurement that defines the number of moles of solute that contribute to the osmotic pressure of a solution. A milliosmole (mOsm) is 1/1,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urine
Urine is a liquid by-product of metabolism in humans and in many other animals. Urine flows from the kidneys through the ureters to the urinary bladder. Urination results in urine being excretion, excreted from the body through the urethra. Cell (biology), Cellular metabolism generates many by-products that are rich in nitrogen and must be clearance (medicine), cleared from the Circulatory system, bloodstream, such as urea, uric acid, and creatinine. These by-products are expelled from the body during urination, which is the primary method for excreting water-soluble chemicals from the body. A urinalysis can detect nitrogenous wastes of the mammalian body. Urine plays an important role in the earth's nitrogen cycle. In balanced ecosystems, urine fertilizes the soil and thus helps plants to grow. Therefore, Reuse of excreta, urine can be used as a fertilizer. Some animals use it to territory (animal)#Scent marking, mark their territories. Historically, aged or fermented urine (kn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urine Osmolality
Urine osmolality is a measure of urine concentration, in which large values indicate concentrated urine and small values indicate diluted urine. Consumption of water (including water contained in food) affects the osmolality of urine. Osmolality is measured by osmometer, which evaluates the freezing point depression of a solution and supplies results as milliosmoles per kilogram of water while specific gravity is measured by colorimetric strips, refractometer, hydrometer and pyknometer. In healthy humans with restricted fluid intake, urine osmolality should be greater than 800 Osmole (unit), mOsm/kg, while a 24-hour urine osmolality should average between 500 and 800 mOsm/kg. Urine osmolality in humans can range from approximately 50 to 1200 mOsm/kg, depending on whether the person has recently drunk a large quantity of water (the lower number) or has gone without water for a long time (the higher number). Plasma osmolality with typical fluid intake often averages approximately ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasma Osmolality
Plasma osmolality measures the body's electrolyte–water balance. There are several methods for arriving at this quantity through measurement or calculation. Osmolality and osmolarity are measures that are technically different, but functionally the same for normal use. Whereas osmolality (with an "l") is defined as the number of osmoles (Osm) of solute per kilogram of solvent (osmol/kg or Osm/kg), osmolarity (with an "r") is defined as the number of osmoles of solute per liter (L) of solution (osmol/L or Osm/L). As such, larger numbers indicate a greater concentration of solutes in the plasma. Measured osmolality (MO) Osmolality can be measured on an analytical instrument called an osmometer. It works on the method of depression of freezing point. Osmolality versus osmolarity Osmolarity is affected by changes in water content, as well as temperature and pressure. In contrast, osmolality is independent of temperature and pressure. For a given solution, osmolarity is slightly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
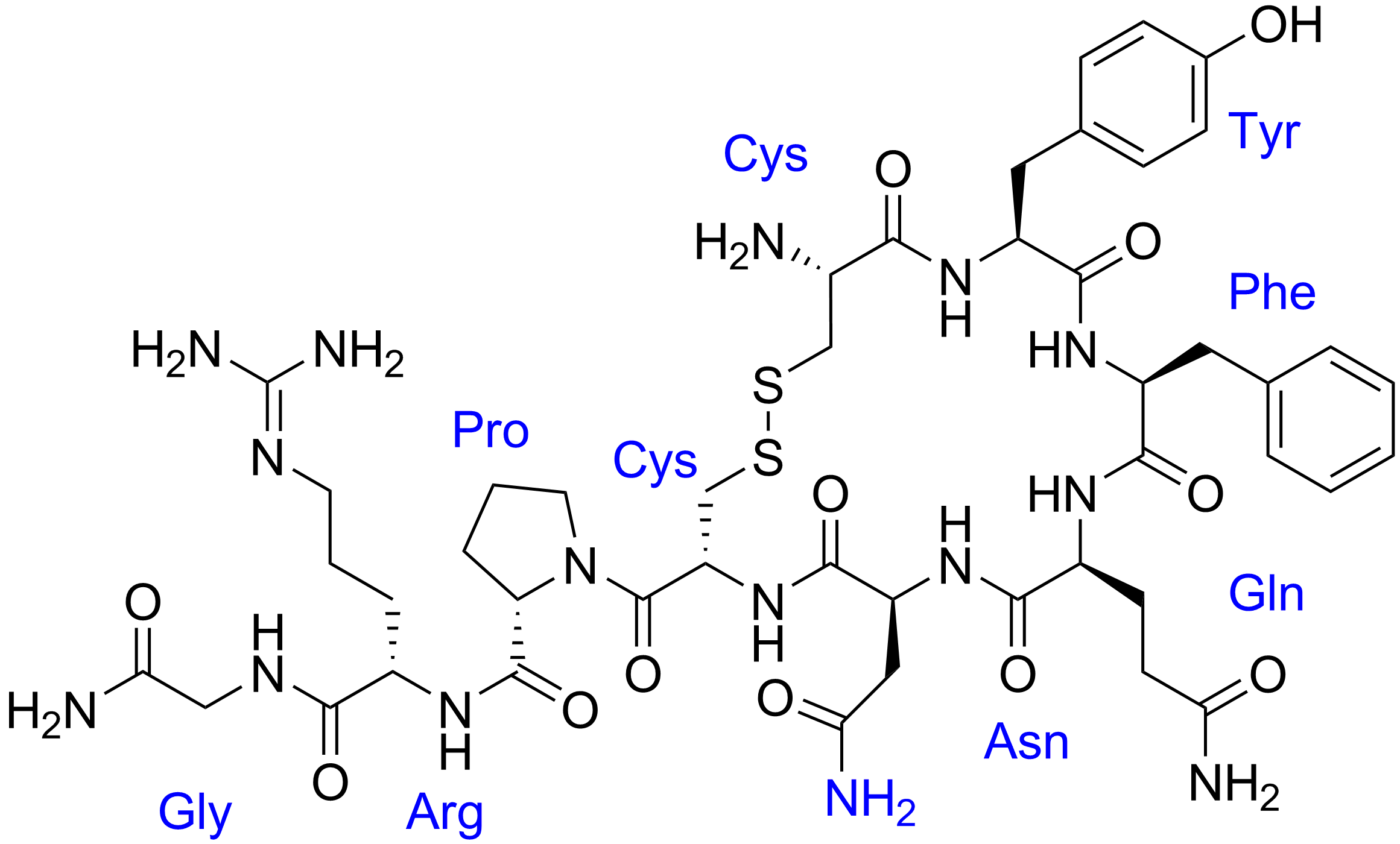
Antidiuretic Hormone
Human vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP) or argipressin, is a hormone synthesized from the AVP gene as a peptide prohormone in neurons in the hypothalamus, and is converted to AVP. It then travels down the axon terminating in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity (hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure. A third function is possible. Some AVP may be released directly into the brain from the hypothalamus, and may play an important role in social behavior, sexual motivation and pair bonding, and maternal responses to stress. Vasopressin induces differentiation of stem cells in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |